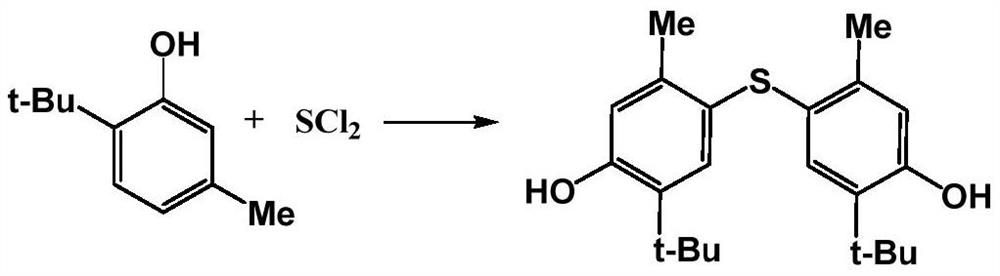
Synthesis method of 4,4'-thiobis(6-tert-butyl-3-methylphenol)
A technology of methyl phenol and synthesis method, applied in chemical instruments and methods, preparation of sulfides, preparation of organic compounds, etc., to achieve the effects of easy recovery and reuse, environmental protection and safety improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 164.2 (1.0 mol) of 2-tert-butyl-5-methylphenol, 169.9 (1.0 mol) of silver nitrate, 253.8 g (1.0 mol) of iodine and 0.7 L of dichloromethane into the reaction kettle, and keep the Stirring, the color of iodine gradually disappears, accompanied by the formation of silver iodide. After 30 minutes, filter and recover the generated silver iodide, wash the filtrate with 1.1L of water and recover the washing water; dry the organic phase with industrial anhydrous magnesium sulfate, add Cu 8.2g, thiourea 38.1g (0.5mol) and triethylamine 200g (about 273ml), then reacted under reflux for 4 hours. Naturally cool to room temperature after the reaction, filter and recover the Cu catalyst, then add water to dilute and wash, separate the water phase, add water to the organic phase and adjust the pH to 6-7 with phosphoric acid, and separate the water phase after standing for stratification. The organic phase was washed once with saturated sodium chloride aqueous solution and water ...
Embodiment 2
[0036] Add 164.2 (1.0 mol) of 2-tert-butyl-5-methylphenol, 220.9 (1.0 mol) of silver trifluoroacetate, 253.8 g (1.0 mol) of iodine and 0.8 L of chloroform into the reaction kettle, and place the Stirring, the color of iodine gradually disappears, accompanied by the formation of silver iodide. After 30 minutes, filter and recover the generated silver iodide, wash the filtrate with 1L of water and recover the washing water; after the organic phase is dried with industrial anhydrous magnesium sulfate, add Cu 8.2g, thiourea 38.1g (0.5mol) and triethylamine 200g (about 273ml), then reacted under reflux for 4 hours. Naturally cool to room temperature after the reaction, filter and recover the Cu catalyst, then add water to dilute and wash, separate the water phase, add water to the organic phase and adjust the pH to 6-7 with phosphoric acid, and separate the water phase after standing for stratification. The organic phase was washed once with saturated sodium chloride aqueous solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com