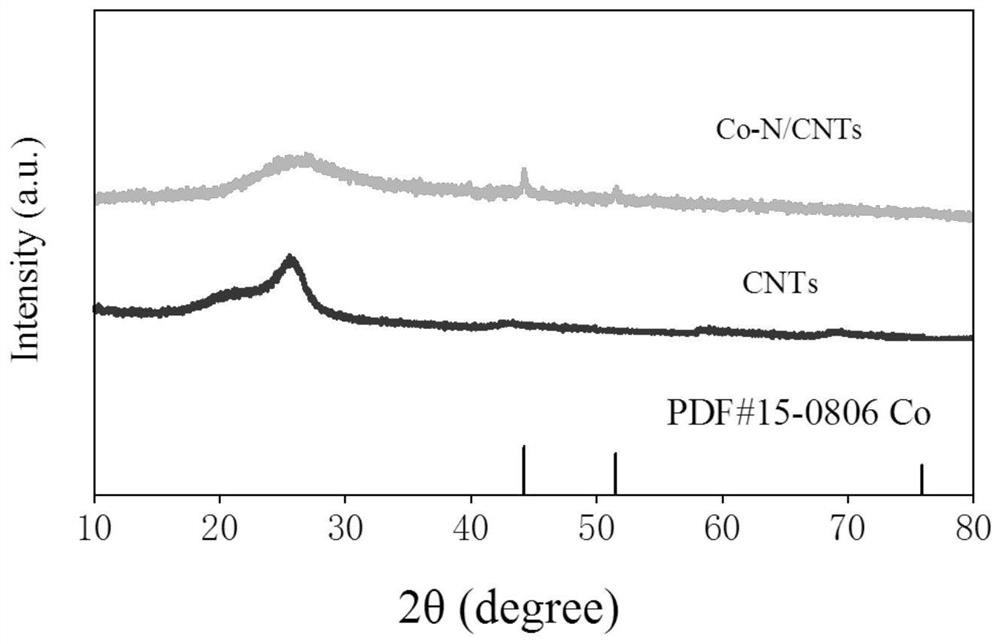
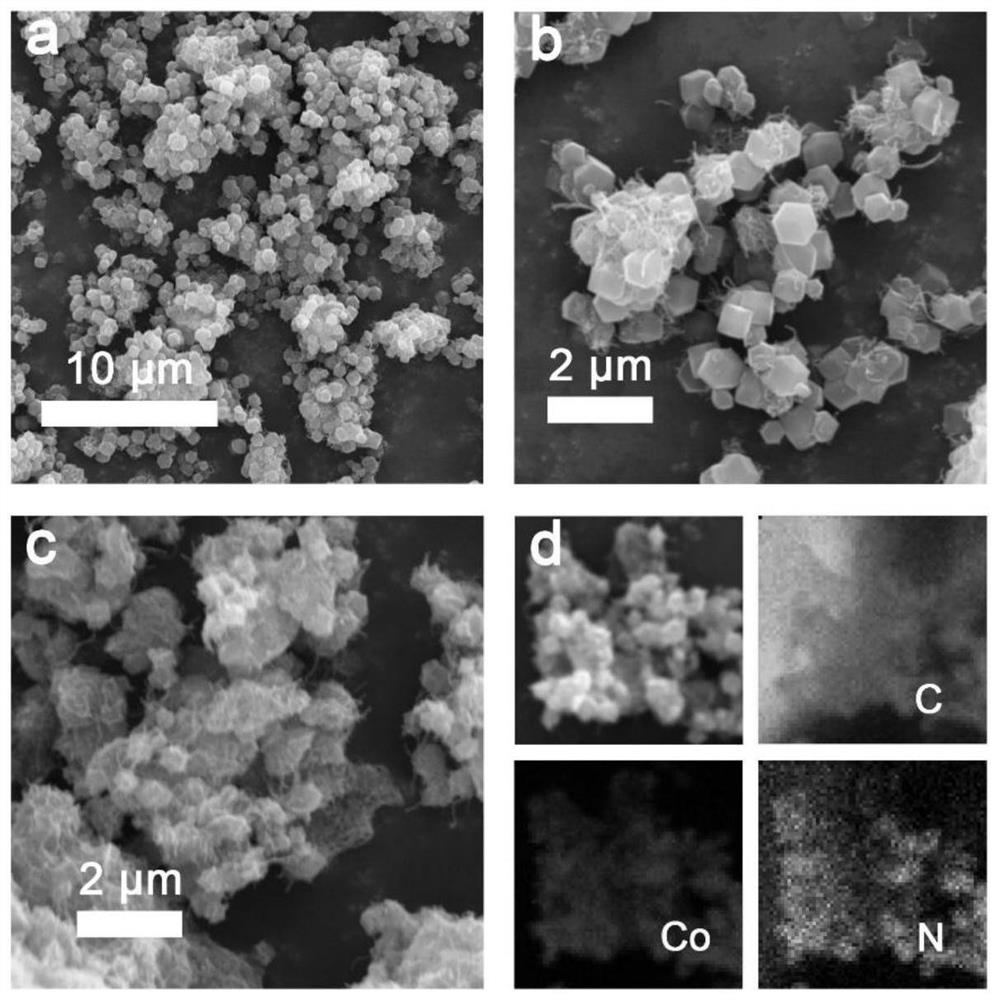
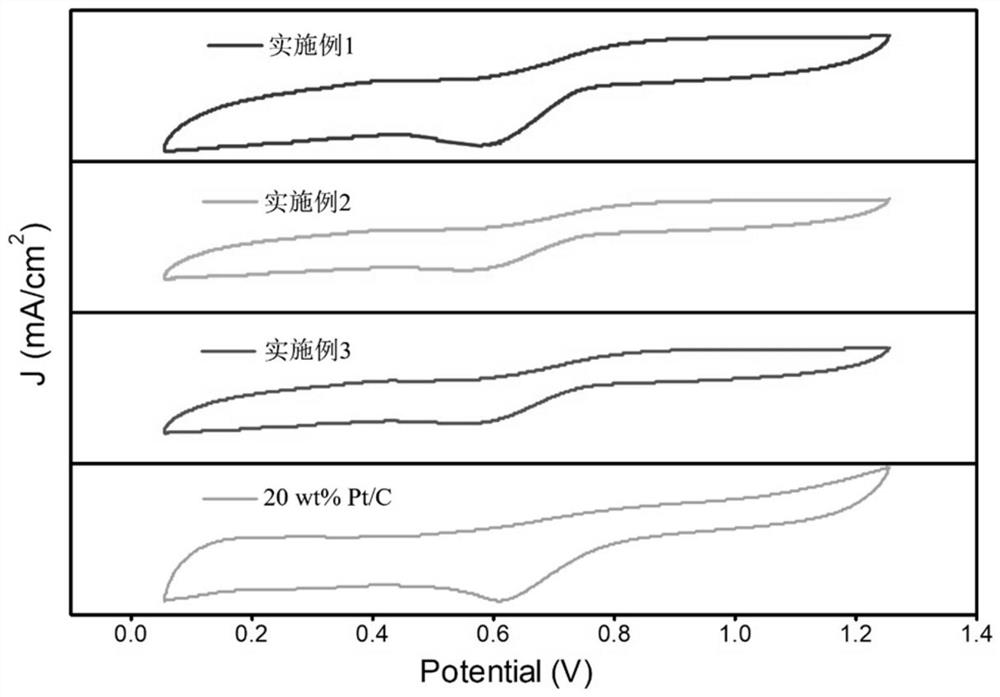
Preparation method of Co-N/CNTs catalytic material, catalytic material obtained through preparation method and application of Co-N/CNTs catalytic material
A catalytic material, co-n technology, applied in biochemical fuel cells, final product manufacturing, sustainable manufacturing/processing, etc., can solve the problems of slow reaction kinetics, restricted MFC performance, insufficient ORR catalyst performance, etc., to achieve easy synthesis , Improve catalytic activity and stability, good oxygen reduction activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A kind of synthetic method of the catalytic material of Co-N / CNTs, comprises the following steps:
[0032] (1) Synthesis of ZIF-67 / CNT: 35 mg of carboxylated multi-walled carbon nanotubes and 175 mg of polyvinylpyrrolidone with a molecular weight of 10,000 were dissolved in 25 mL of methanol and ultrasonically oscillated for 0.5 h;
[0033] (2) Add 222 mg of cobalt chloride to the carbon nanotube dispersion obtained in step (1), and continue stirring for 1 hour;
[0034] (3) Dissolve 765mg of organic ligand 2-methylimidazole in 25mL of methanol solution, then add dropwise into the above mixed solution, then put it into a high-temperature reactor at 100°C, and react for 24h;
[0035] (4) The obtained product was filtered and washed several times with methanol and deionized water, then dried and ground to obtain a powder;
[0036] (5) The precursor powder prepared in step (4) was carbonized at 800°C for 2 hours under an inert atmosphere, and the heating rate was 5°C·min ...
Embodiment 2
[0041] A kind of synthetic method of the catalytic material of Co-N / CNTs, comprises the following steps:
[0042] (1) Synthesis of ZIF-67 / CNT: 35 mg of carboxylated multi-walled carbon nanotubes and 140 mg of polyvinylpyrrolidone with a molecular weight of 10,000 were dissolved in 35 mL of methanol and ultrasonically oscillated for 2.0 h;
[0043] (2) Add 222 mg of cobalt chloride to the carbon nanotube dispersion obtained in step (1), and continue stirring for 1 hour;
[0044] (3) Dissolve 560 mg of organic ligand 2-methylimidazole in 35 mL of methanol solution, then add it dropwise to the above mixed solution, and then put it in a high-temperature reactor at 100 ° C for 36 hours;
[0045] (4) The obtained product was filtered and washed several times with methanol and deionized water, then dried and ground to obtain a powder;
[0046] (5) The precursor powder prepared in step (4) was carbonized at 700 °C for 3 hours under an inert atmosphere, and the heating rate was 2.5 °C...
Embodiment 3
[0048] A kind of synthetic method of the catalytic material of Co-N / CNTs, comprises the following steps:
[0049] (1) Synthesis of ZIF-67 / CNT: 35 mg of carboxylated multi-walled carbon nanotubes and 105 mg of polyvinylpyrrolidone with a molecular weight of 10,000 were dissolved in 40 mL of ethanol and ultrasonically oscillated for 0.5 h;
[0050] (2) Add 222 mg of cobalt chloride to the carbon nanotube dispersion obtained in step (1), and continue stirring for 1 hour;
[0051] (3) Dissolve 315 mg of organic ligand 2-methylimidazole in 40 mL of methanol solution, then add it dropwise to the above mixed solution, and then put it in a high-temperature reactor at 110 ° C for 36 hours;
[0052] (4) The obtained product was filtered and washed several times with methanol and deionized water, then dried and ground to obtain a powder;
[0053] (5) The precursor powder prepared in step (4) was carbonized at 600°C for 4 hours under an inert atmosphere, and the heating rate was 1°C·min ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com