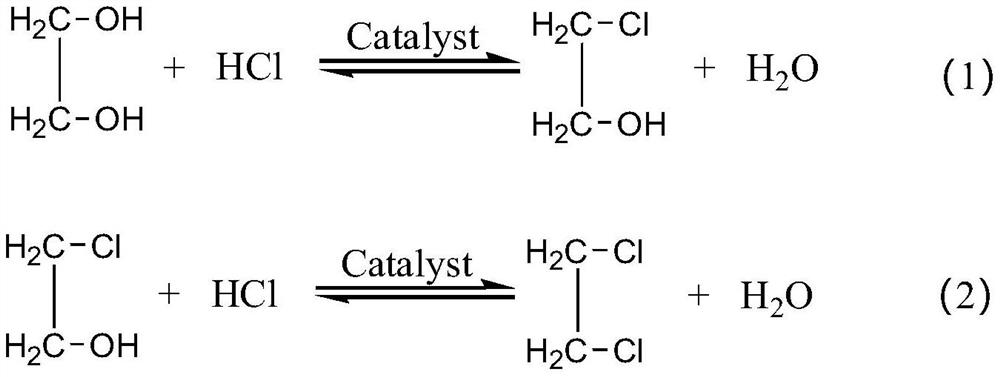
Method for producing chloroethanol and dichloroethane through ethylene glycol chlorination
A technology of ethylene dichloride and ethylene glycol, applied in chemical instruments and methods, bulk chemical production, organic chemistry, etc., can solve problems such as high production cost of ethylene oxide, difficulty in catalyst recovery, difficulty in transportation, etc., and achieve Good catalytic stability, improved selectivity and yield, good catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The method for preparing chloroethanol and ethylene dichloride by chlorination of ethylene glycol described in this embodiment comprises the following steps: take 1000 kg of ethylene glycol and 5 kg of active catalyst and place them in a reactor for mixing, and control the temperature of the reaction system at 60°C, followed by 75.87Nm 3 The flow rate of / h is passed through excess HCl gas (the molar ratio of the total flow rate of HCl gas to ethylene glycol is 1.05:1) for chlorination reaction for 5h, and after the tail gas is condensed, it is absorbed by NaOH solution to remove unreacted HCl, after the reaction, the product is filtered to recover the active catalyst, washed, dried and activated for recycling.
[0031] In the method of the present embodiment, described active catalyst uses Co 2 o 3 · MnO 2 · Fe 2 o 3 As the active component, with Zn(NO 3 ) 2 It is an active auxiliary agent, and it is prepared by impregnating the surface of cordierite ceramic bal...
Embodiment 2
[0036] The method for preparing chloroethanol and ethylene dichloride by chlorination of ethylene glycol described in this embodiment comprises the following steps: take 1000 kg of ethylene glycol and 5 kg of active catalyst and place them in a reactor for mixing, and control the temperature of the reaction system at 70°C, followed by 47.42Nm 3 The flow rate of / h is passed through excess HCl gas for chlorination reaction for 8h, and the tail gas is condensed and then absorbed by 5% NaOH solution to remove unreacted HCl. After the reaction, the product is filtered to recover the active catalyst, washed and dried , After activation, it can be recycled.
[0037] In the method of the present embodiment, described active catalyst uses Co 2 o 3 · MnO 2 · Fe 2 o 3 As the active component, with Zn(NO 3 ) 2 It is an active auxiliary agent, and it is prepared by impregnating the surface of activated alumina in the form of slurry. The specific preparation steps include:
[0038]...
Embodiment 3
[0042] The method for preparing chloroethanol and ethylene dichloride by chlorination of ethylene glycol described in this example comprises the following steps: take 1000 kg of ethylene glycol and 10 kg of active catalyst and mix them in a reactor, and control the temperature of the reaction system at 80°C, followed by 63.23Nm 3 The flow rate of / h is passed through excess HCl gas for chlorination reaction for 6h, and the tail gas is condensed and then absorbed by NaOH solution to remove unreacted HCl. After the reaction, the product is filtered to recover the active catalyst, washed, dried and activated. After that, it is recycled.
[0043] In the method of the present embodiment, described active catalyst uses Co 2 o 3 · MnO 2 · Fe 2 o 3 As the active component, with Zn(NO 3 ) 2 It is an active auxiliary agent, and it is prepared by impregnating the surface of cordierite honeycomb ceramics in the form of slurry. The specific preparation steps include:
[0044] (1) T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com