Immune vesicle maytansine conjugate as well as preparation method and application thereof
A technology of maytansinoids and maytansinoids, applied in drug combinations, pharmaceutical formulations, nano-drugs, etc., can solve the problems of limited research, large antibody dosage, and low drug content, and achieve good biocompatibility, high efficiency and stability The effect of the package effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Reversible cross-linked and biodegradable vesicular maytansine conjugates (N 3 -Ps-DM1) Preparation
[0044] N 3 -Ps-DM1 is prepared by a solvent replacement method, wherein the dithiolane ring in the hydrophobic segment is chemically bonded to DM1 through sulfhydryl-sulfur-sulfur exchange, and the drug is coupled in the hydrophobic membrane of the vesicle. N 3 -Ps-DM1 by N 3 - PEG-P (TMC-DTC) and PEG-P (TMC-DTC) co-assembled and coupled to DM1, wherein N 3 - The content of PEG-P(TMC-DTC) is 1~10 wt.%. Specifically, to contain 2 wt.% N 3 -N of PEG-P (TMC-DTC) 3 - Take the preparation of Ps-DM1 as an example, weigh 0.72 mgN 3 - PEG-P(TMC-DTC) and 35.28 mg PEG-P(TMC-DTC) were dissolved in DMF (total polymer concentration 40 mg / mL); 0.4 mL of DM1 in DMF (10 mg / mL) was added Mix well with 7.7 mL of PB (pH 7.4, 10 mM), inject 0.9 mL of polymer solution into it under stirring, stir for 3 minutes, then place at 37 ºC for 12 hours. After dialysis (MWCO = 3.5 ...
Embodiment 2
[0046] Example 2 Preparation of mAb-directed immune vesicle maytansine conjugate (Ab-Ps-DM1)
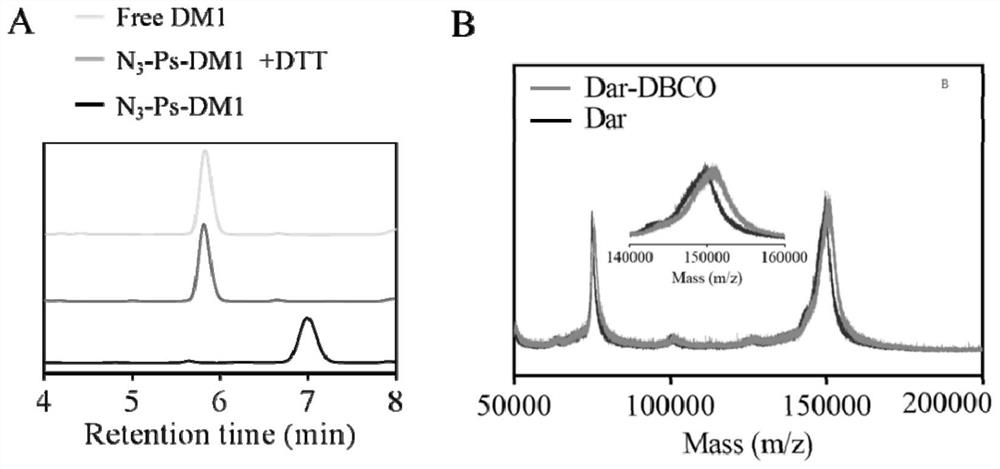
[0047] Ab-Ps-DM1 via DM1 nanomedicine in azide-functionalized polymersomes (N 3 -Ps-DM1) surface post-modified dibenzocyclooctyne functionalized monoclonal antibody (Ab-DBCO). First, in order to bind the mAb efficiently, the N 3 -Ps-DM1 was concentrated from 4 mg / mL to 19.8 mg / mL. Concentrated N 3 -Ps-DM1 has a particle size of 48 nm and a PDI of 0.16. The long-term storage stability of N3-Ps-DM1 was characterized by monitoring its particle size, particle size distribution and drug leakage during storage at 4 ºC for 180 days. -Ps-DM1 exhibited little change in particle size and particle size distribution (within 3 nm), and no DM1 leakage, indicating its best long-term storage stability. After treatment with 10 mMDTT, DM1 was rapidly released in the free form ( figure 1 A). In addition, N 3 -The release of Ps-DM1 at 37 ºC in PBS containing 0.3% Tween 80, no free DM1 was detect...
Embodiment 3
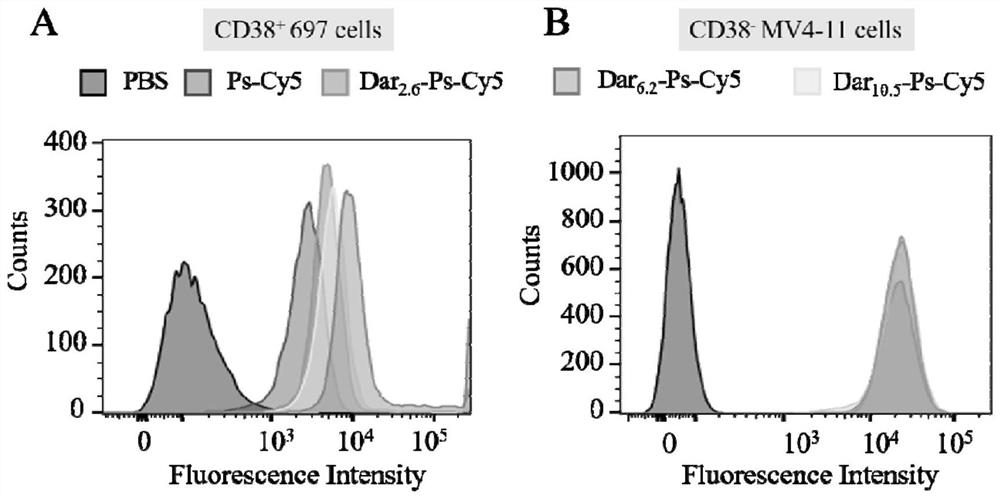
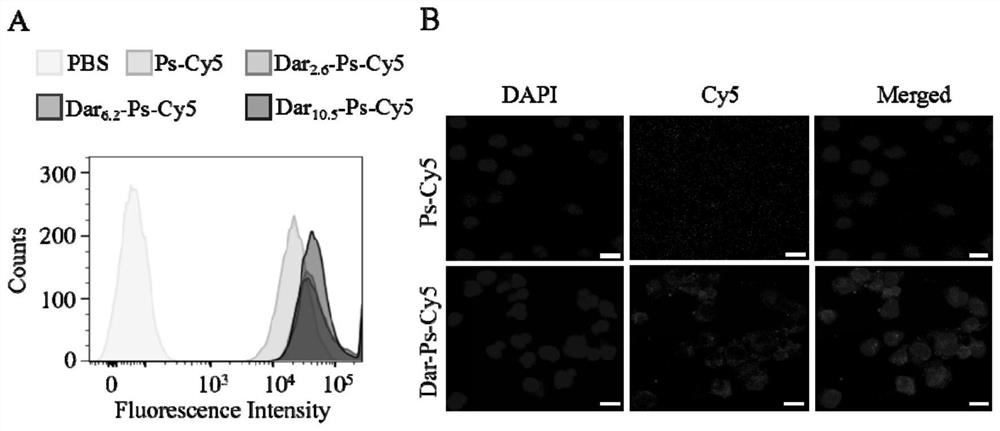
[0056] Example 3 Endocytosis of Dar-Ps-DM1
[0057] Since DM1 itself has no fluorescence, Cy5-labeled polymersomes (Dar-Ps-Cy5) were used to study the effect of Dar-Ps-Cy5 with different Dar densities on 697 acute lymphoid leukemia by flow cytometry and confocal laser microscopy (CLSM). cells, LP-1 multiple myeloma cells and MV4-11 leukemia cells. Ps-Cy5 through 2 wt.% N 3 -Polymer co-assembly of PEG-P(TMC-DTC), 20 wt.% PEG-P(TMC-DTC)-Cy5 and 78 wt.% PEG-P(TMC-DTC) was prepared. The details are as follows: Weigh 0.32 mg N 3 - PEG-P(TMC-DTC), 3.2 mg PEG-P(TMC-DTC)-Cy5, 12.48 mg PEG-P(TMC-DTC) were dissolved in DMF (40 mg / mL), and the three polymers were mixed well Then, inject into 3.6 mL PB (pH 7.4, 10 mM) with stirring. After stirring for 3 min, dialyze (MWCO = 1 kDa) against PB (pH 7.4, 10 mM) to remove the organic solvent and concentrate to a polymer concentration of 17 mg / mL. The preparation method of Dar-Ps-Cy5 is similar to Dar-Ps-DM1, setting Dar-DBCO and N 3 The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com