Oral megestrol acetate suspension and preparation method thereof
A technology of megestrol acetate and suspension, which is applied in the field of medicine, can solve problems such as poor physical stability, and achieve the effects of increasing dissolution rate, improving efficiency, and shortening the preparation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
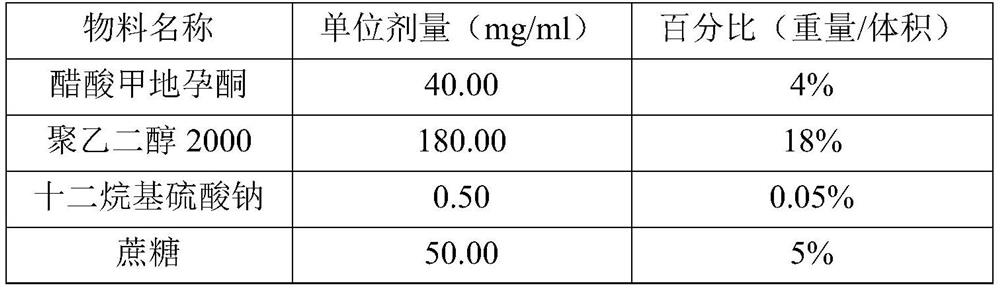
[0070] Material name Unit dose (mg / ml) Percentage (weight / volume) megestrol acetate 40.00 4% polyethylene glycol 2000 180.00 18% Sodium dodecyl sulfate 0.50 0.05% sucrose 50.00 5% xanthan gum 1.8 0.18% sodium benzoate 2.00 0.2% citric acid 2.44 0.244% sodium citrate 0.17 0.017% lemon zest 0.8 0.08% purified water Appropriate amount Appropriate amount
[0071] Process Description:
[0072] Step 1 (preparation of the first solution)
[0073] Add part of the purified water into the stirring tank (M), add the prescribed amount of polyethylene glycol 2000, heat to 50°C, stir to dissolve, the stirring speed is 20rpm, and cool down to ≤35°C.
[0074] Add the prescribed amount of sodium lauryl sulfate, stir to dissolve.
[0075] Add the prescribed amount of megestrol acetate, stir to disperse evenly, and the stirring speed is 55rpm.
[0076] Step 2 (preparation of the second solution solution) ...
Embodiment 2
[0090] Xanthan gum and sucrose are pre-mixed in equal increments and then added to the suspension system (homogeneous 3600rpm, time 120 minutes)
[0091] Material name Unit dose (mg / ml) Percentage (weight / volume) megestrol acetate 40.00 4% polyethylene glycol 2000 180.00 18% Sodium dodecyl sulfate 0.50 0.05% sucrose 50.00 5% xanthan gum 1.8 0.18% sodium benzoate 2.00 0.2% citric acid 2.44 0.244% sodium citrate 0.17 0.017% lemon zest 0.8 0.08% purified water Appropriate amount Appropriate amount
[0092] Process Description:
[0093] Step 1 (preparation of the first solution)
[0094] Add part of purified water into the stirring tank (M), add the prescribed amount of polyethylene glycol 2000, heat to 50°C, stir at 20rpm, and cool down to ≤35°C.
[0095] Add the prescribed amount of sodium lauryl sulfate, stir to dissolve.
[0096] Add the prescribed amount of megestrol acetate, stir and ...
Embodiment 3
[0111] Xanthan gum and sucrose are pre-mixed in equal increments and then added to the suspension system (homogeneous speed 3600rpm, time 90 minutes)
[0112]
[0113]
[0114] Process Description:
[0115] Step 1 (preparation of the first solution)
[0116] Add part of purified water into the stirring tank (M), add the prescribed amount of polyethylene glycol 2000, heat to 50°C, stir at 20rpm, and cool down to ≤35°C.
[0117] Add the prescribed amount of sodium lauryl sulfate, stir to dissolve.
[0118] Add the prescribed amount of megestrol acetate, stir and disperse evenly. Stirring speed 55rpm.
[0119] Step 2 (preparation of the second solution solution)
[0120] Add an appropriate amount of water into the stirring tank (N), heat to 40°C, add the prescribed amount of sodium benzoate, stir to dissolve, and the stirring speed is 20rpm.
[0121] Pre-disperse the prescription amount of xanthan gum and sucrose in equal increments before adding, then add the disperse...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com