Synthesis method of amino-terminated polyolefin
A synthesis method and terminal amination technology, applied in the field of synthesis of terminal amination polyolefin, can solve the problems of high cost of reagents, cumbersome synthesis route, etc., and achieve the effects of simple method, low cost, and promotion of high value-added recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The present invention provides a kind of synthetic method of aminated polyolefin, comprising:
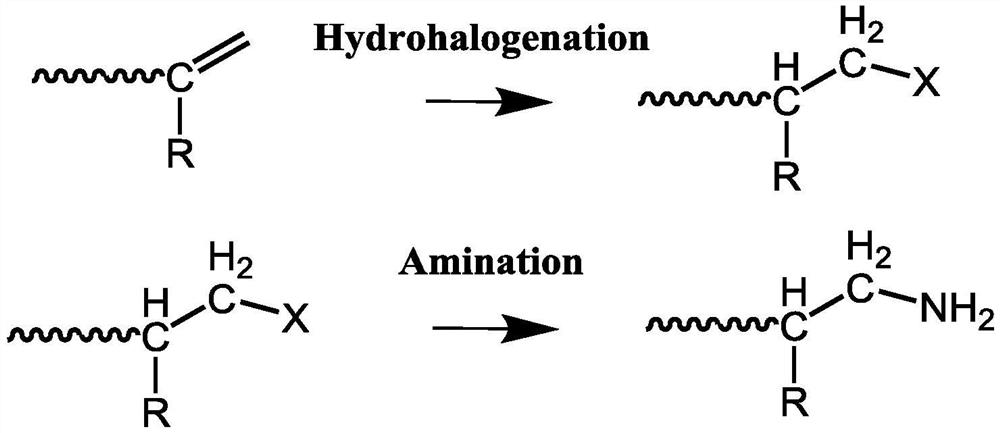
[0025] A) mixing the hydrohalogenation reagent and the polyolefin thermal degradation product, stirring, and reacting to obtain a terminal halogenated polyolefin macromer;
[0026] B) Mix terminal halogenated polyolefin macromonomer with solvent, feed ammonia gas, stir and react to obtain terminal aminated polyolefin.
[0027] The method for synthesizing end-aminated polyolefins provided by the invention firstly mixes a hydrohalogenation reagent and a thermal degradation product of polyolefins.
[0028] The thermal degradation product of polyolefin in the present invention is a thermal degradation product of polyolefin; the heating temperature is preferably 250°C-450°C. The present invention does not limit the thermal degradation time, which may be 0.5h to several hours.
[0029] The polyolefin of the present invention includes but not limited to polyethylene, polypropylene...
Embodiment 1
[0050] Embodiment 1 (taking polypropylene as example)
[0051] In a 500ml flask, add 100g of polypropylene thermal degradation product (Mn: 2800mol / g) into 300ml of hexane, then add 0.65 parts by weight of hydrogen bromide glacial acetic acid solution, stir at room temperature for 12h, then suction filter , washed with ethanol, and dried to obtain a brominated polypropylene macromonomer. Subsequently, the obtained terminal brominated polypropylene macromonomer was added into a high-pressure reactor containing 500 ml of tetrahydrofuran, and then saturated with ammonia gas, and then the reactor was heated to 150° C. and stirred for 10 h. Then, cool to room temperature, filter with suction, wash with ethanol and distilled water, and dry.
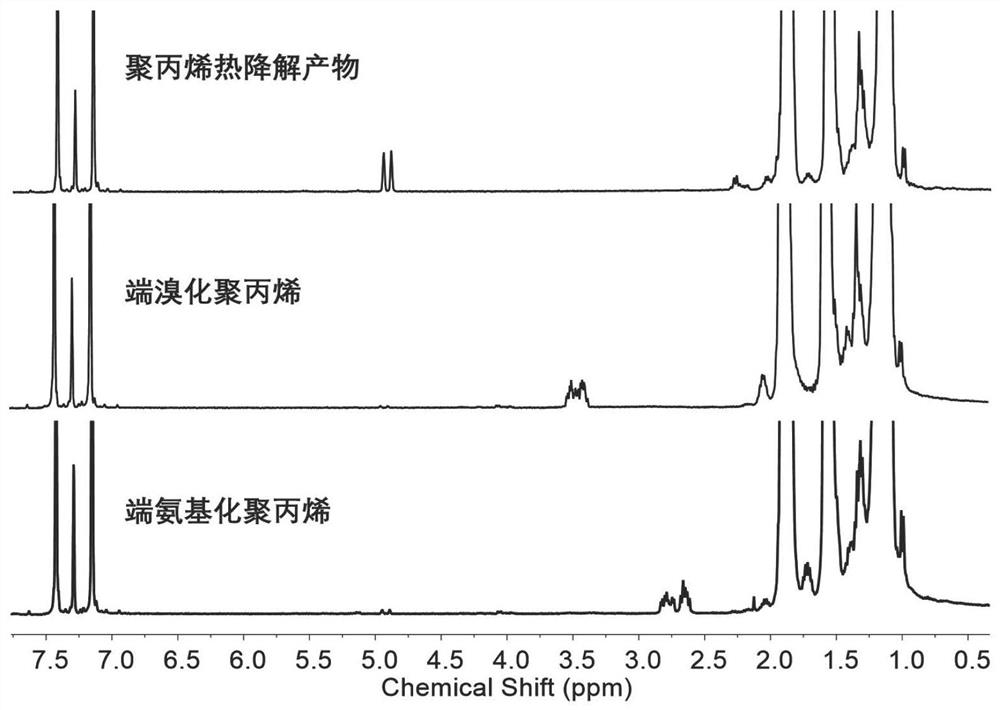
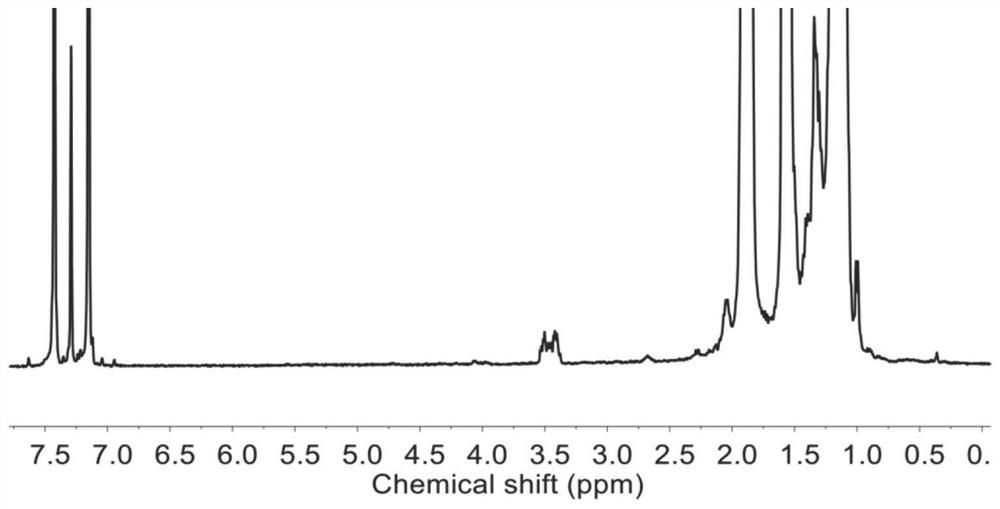
[0052] Carry out NMR analysis to the polymer prepared in this embodiment, figure 1 The H NMR spectra of raw materials for functionalized polypropylene, brominated polypropylene, and aminated polypropylene were prepared for this example. NMR ...
Embodiment 2
[0053] Embodiment 2 (taking polyethylene as example)
[0054] In a 500ml flask, add 100g of polyethylene thermal degradation product (Mn: 1474mol / g) and 300ml of toluene, then pass in hydrogen bromide gas, react at room temperature for 12h, and replace the hydrogen bromide gas with nitrogen gas exchange. Subsequently, the obtained terminal brominated polyethylene macromonomer was added into a high-pressure reactor containing 500 ml of tetrahydrofuran, saturated with ammonia gas, and then the reactor was heated to 100° C. and stirred for 10 h. Then, cool to room temperature, filter with suction, wash with ethanol and distilled water, and dry. NMR results show that the product is brominated, the degree of amination is 80%, and the overall functionality is 40-50%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com