Method for synthesizing nuclear grade boric acid through hydrolysis of boron trifluoride
A technology of boron trifluoride and synthetic nuclei, applied in the direction of fluorine/hydrogen fluoride, boron oxide, etc., can solve the problems of high cost, insufficient purity and complicated process of electrolytic electrodialysis, achieve significant economic and environmental benefits, and improve purity and the effect of the yield and the simplicity of the synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The method for synthesizing nuclear grade boric acid by hydrolysis of boron trifluoride comprises the following steps:
[0022] (1) React boron trifluoride with a boron 10 abundance of more than 96% and water, set nitrogen as the protective gas, the molar ratio of boron trifluoride to water is 4:3, the initial reaction temperature is 0°C, and the reaction time In 25 hours, the boric acid aqueous solution was collected at the bottom of the reactor, and the generated hydrogen fluoride was absorbed with water to obtain a hydrofluoric acid product, or absorbed with an alkali solution to obtain a fluoride salt solution;
[0023] (2) recrystallize and purify the boric acid obtained in step (1), set the crystallization temperature to 10° C., set the solution concentration to 30%, and dry the obtained boric acid crystals after the crystallization is completed.
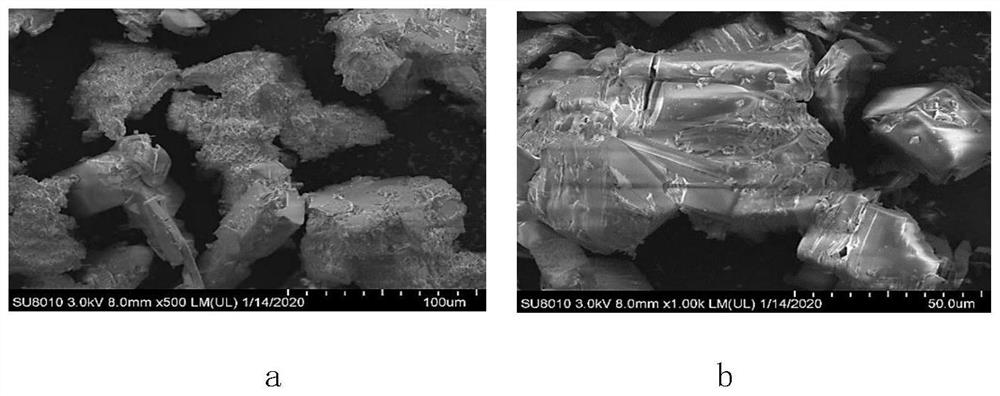
[0024] Boric acid (B(OH) 3 ) with a particle size of 350-700 μm.
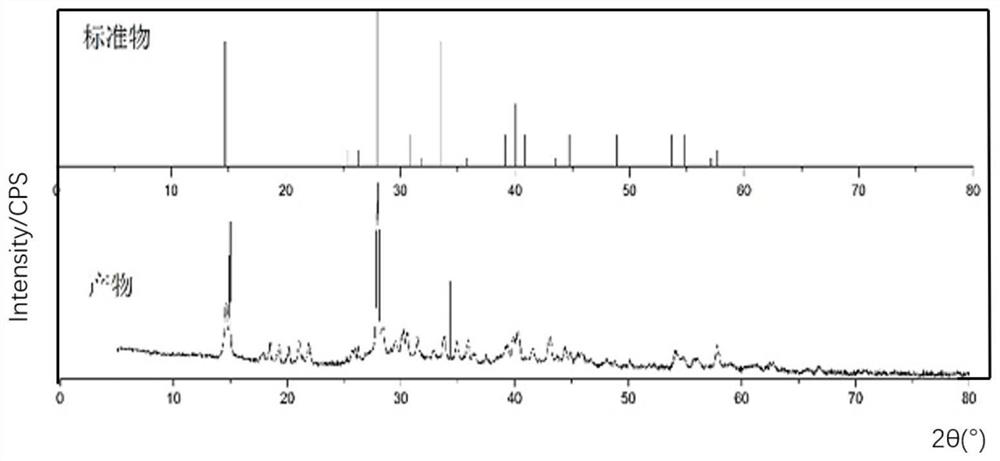
[0025] Boric acid (B(OH) 3 ) with a purity of 9...
Embodiment 2
[0028] The method for synthesizing nuclear grade boric acid by hydrolysis of boron trifluoride comprises the following steps:
[0029] (1) React boron trifluoride with a boron 10 abundance of more than 96% and water, set nitrogen as the protective gas, the molar ratio of boron trifluoride to water is 2:3, the initial reaction temperature is 30°C, and the reaction time In 25 hours, the boric acid aqueous solution was collected at the bottom of the reactor, and the generated hydrogen fluoride was absorbed with water to obtain a hydrofluoric acid product, or absorbed with an alkali solution to obtain a fluoride salt solution;
[0030] (2) recrystallize and purify the boric acid obtained in step (1), set the crystallization temperature to 10° C., set the solution concentration to 30%, and dry the obtained boric acid crystals after the crystallization is completed.
[0031] Boric acid (B(OH) 3 ) with a particle size of 400-800 μm.
[0032] Boric acid (B(OH) 3 ) with a purity of ...
Embodiment 3
[0035] The method for synthesizing nuclear grade boric acid by hydrolysis of boron trifluoride comprises the following steps:
[0036] (1) React boron trifluoride with a boron 10 abundance of more than 96% and water, set nitrogen as the protective gas, the molar ratio of boron trifluoride to water is 2:3, the initial reaction temperature is 5°C, and the reaction time In 15 hours, the boric acid aqueous solution was collected at the bottom of the reactor, and the generated hydrogen fluoride was absorbed by water to obtain hydrofluoric acid product, or absorbed by alkali solution to obtain a fluoride salt solution;
[0037] (2) recrystallize and purify the boric acid obtained in step (1), set the crystallization temperature to 10° C., set the solution concentration to 30%, and dry the obtained boric acid crystals after the crystallization is completed.
[0038] Boric acid (B(OH) free) 3 )produce.
[0039] Substitute the reaction time of Example 3 for 15 hours with a reaction t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com