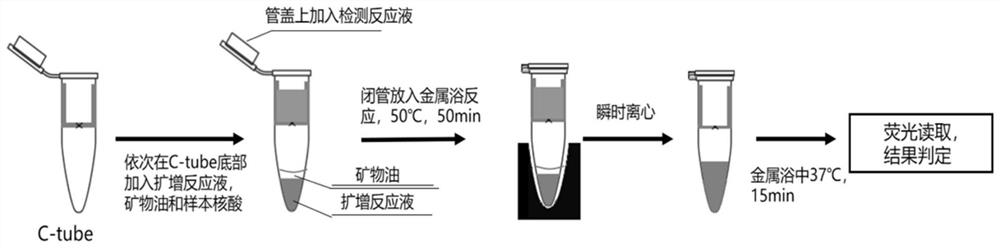
Novel coronavirus nucleic acid detection kit
A technology for detection kits and coronaviruses, applied in the determination/inspection of microorganisms, biochemical equipment and methods, microorganisms, etc., can solve problems such as complex operations, unsuitable diagnostic detection methods, and long time consumption, and achieve high sensitivity and difficulty Contamination, high specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
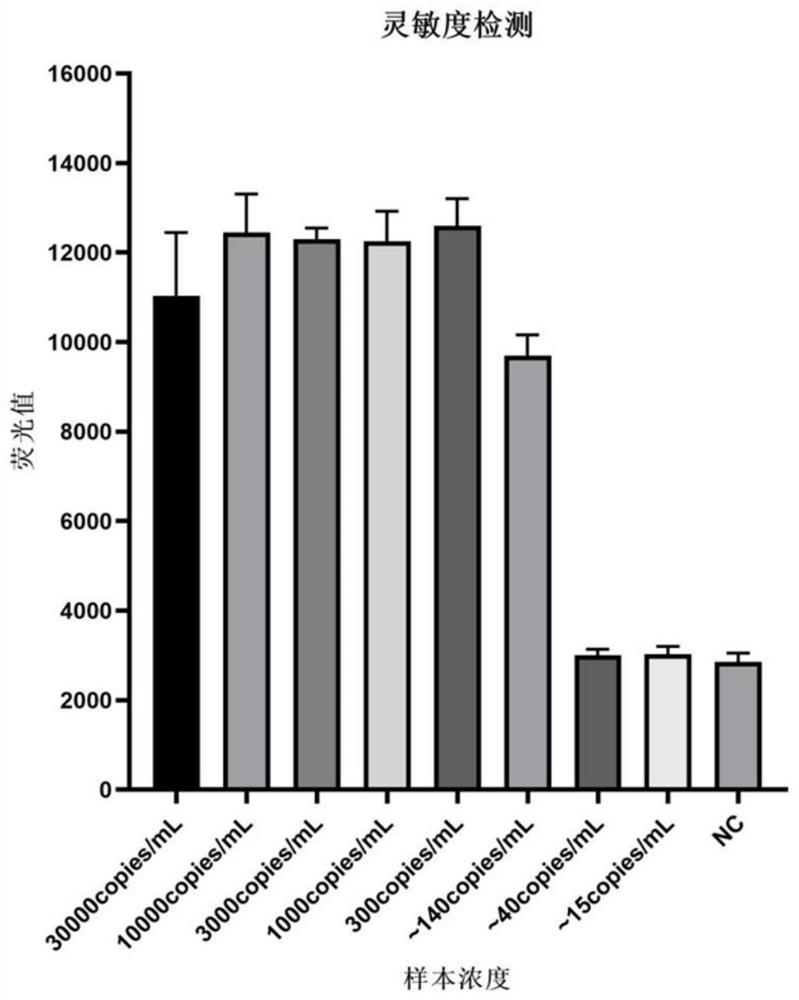
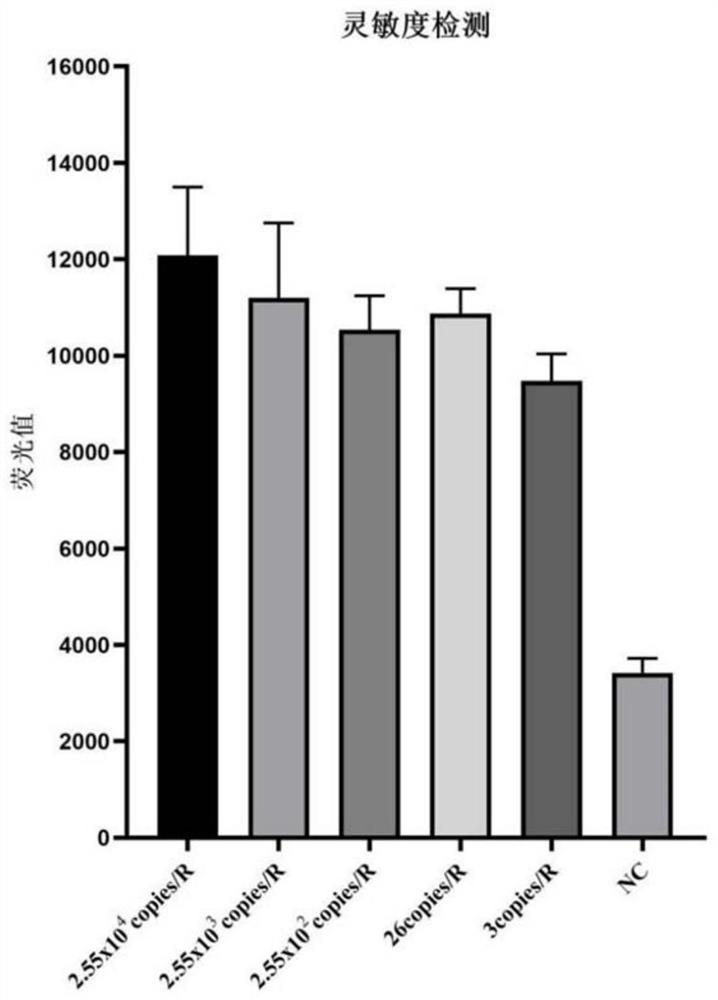
[0081] Embodiment 1: the detection of kit sensitivity of the present invention
[0082] 1. Preparation for detection of sample nucleic acid:
[0083] 1) Take the concentration of 30,000 copies / mL after the inactivation of the culture of the new coronavirus as the S1 sample, and sequentially dilute it with PBS according to a ratio of 1:3, and then the S2 sample is 10,000 copies / mL, the S3 sample is about 3,000 copies / mL, and the S4 sample is about 3000 copies / mL. 1000copies / mL, S5 sample is about 300copies / mL, S6 sample is about 140copies / mL, S7 sample is about 40copies / mL, S8 sample is about 15copies / mL, NC sample is PBS solution. After nucleic acid extraction, the above S1-S8 samples and NC samples become the nucleic acid sample solution to be detected by the kit in this embodiment. The process of nucleosomes uses the pathogenic biological nucleic acid extraction kit (article number MD002) provided by our company (Hangzhou Jieyi Biotechnology Co., Ltd.).
[0084] 2) Through...
Embodiment 2
[0111] Embodiment 2: the detection of kit specificity of the present invention
[0112] 1. Preparation for detection of sample nucleic acid:
[0113] 1) The inactivated sample of the new coronavirus culture is used as the positive control PC sample, and the common respiratory or lung infection pathogen sample is used as the specific interference sample for testing. These pathogen samples include Legionella pneumophila L. pneumoniae, Klebsiella pneumoniae K.pneumoniae, Streptococcus pneumoniae S.pneumoniae, Haemophilus influenzae H.influenzae, Mycoplasma pneumoniae M.pneumoniae, Chlamydia pneumoniae C.pneumoniae, B.pertussis pertussis, human coronavirus-OC43 hCoV -OC43, human coronavirus-NL63 hCoV-NL63, human coronavirus-HKU-1hCoV-HKU-1, human coronavirus-229E hCoV-229E, MERS pseudovirus MERS Armored RNA, adenovirus type 3 Adenovirus type 3, parainfluenza Type 2 Parainfluenza type 2, Respiratory Syncytial Virus A Type RSV-A, Avian Influenza Virus H7N9 Influenza A H7N9, Avian I...
Embodiment 3
[0116] Embodiment 3: the detection of the kit of the present invention to the clinical throat swab sample
[0117] 1. Preparation for detection of sample nucleic acid:
[0118] 1) Based on the throat swab samples of patients clinically diagnosed as new coronavirus infection, take 8 nucleic acid sample solutions extracted from positive throat swab samples, numbered P1, P2, P3, P4, P5, P6, P7, P8 ; 5 nucleic acid sample solutions extracted from negative throat swab samples, numbered N1, N2, N3, N4, N5, as nucleic acid samples for detection. The above throat swab nucleic acid sample solutions are all nucleic acid sample solutions obtained from clinical throat swab samples after nucleic acid extraction with the QIAamp Viral RNA Mini Kit Viral RNA Extraction Kit (Product No. 52904) from QIAGEN.
[0119] 2. The specific implementation steps of amplification and detection in this embodiment, the determination and interpretation of the detection results are the same as those in Examp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com