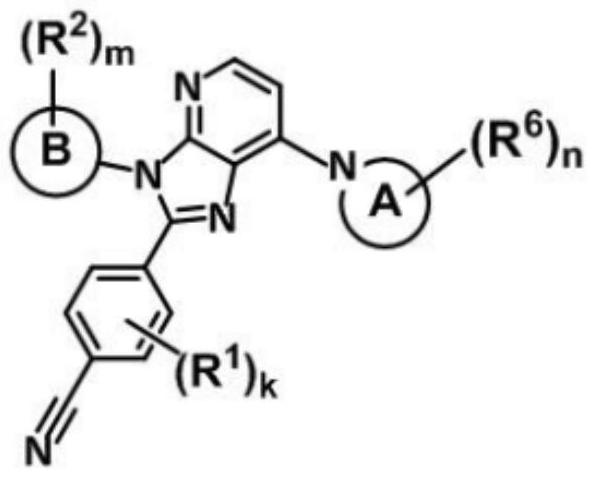
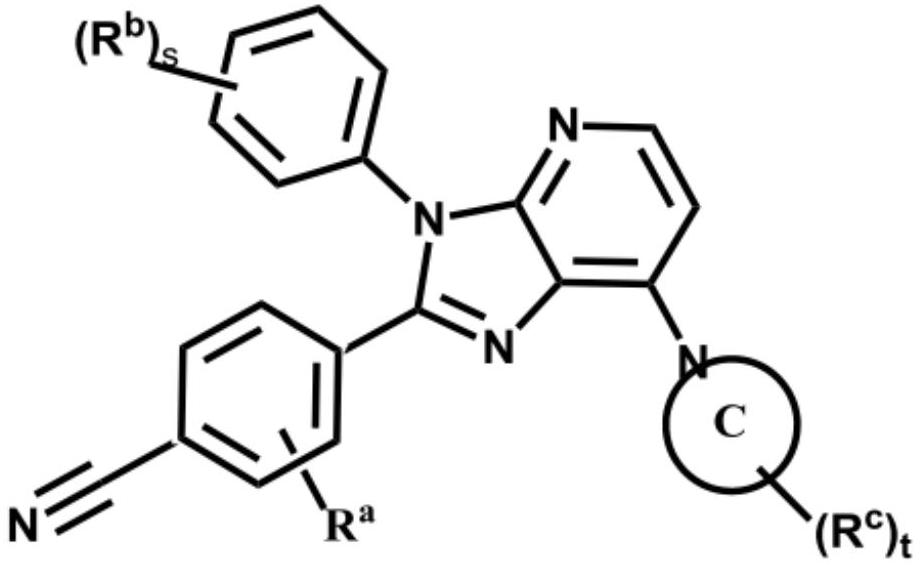
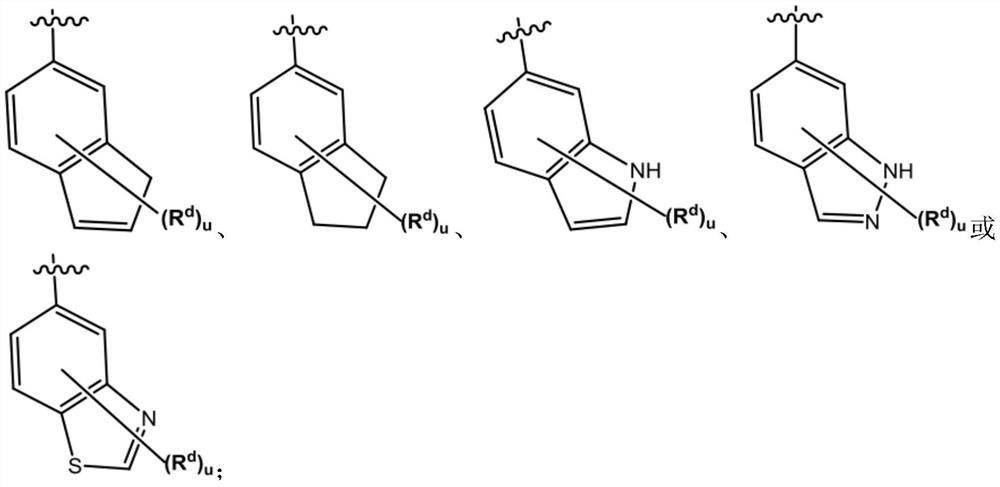
Imidazopyridine derivative compounds and use of same
A compound and solvate technology, applied in the field of imidazopyridine derivative compounds, can solve the problem of not showing enough
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0237] The abbreviations used in the following preparations, preparation methods and examples indicate respectively:
[0238] Protecting group (PG): protecting group
[0239] Boc-: tertiary butoxycarbonyl
[0240] POCl 3 : Phosphoryl chloride
[0241] NaCO 3 :Sodium carbonate
[0242] Ce 2 CO 3 : cesium carbonate
[0243] Na 2 SO 4 : sodium sulfate
[0244] MPLC: medium pressure liquid chromatography (medium pressure liquid chromatography)
[0245] NET 3 (TEA): Triethylamine
[0246] TLC: thin layer chromatography
[0247] PD 2 (dba) 3 : Tris(dibenzylideneacetone)dipalladium(0)
[0248] Xantphos: 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene
[0249] FeCl 3 : Iron(III) chloride
[0250] Celite: diatomaceous earth
[0251] CF 3 CO 2 H: Trifluoroacetate
[0252] N,N-Dimethylformamide (N,N-Dimethyl formamide): Dimethylformamide
[0253] MeOH: Methanol
[0254] EtOH: ethanol
[0255] n-Hexane (n-Hexane): hexane
[0256] Ethyl acetate (EA): ethyl acetat...
preparation example 1
[0282] Preparation Example 1: Preparation of 4-cyclopropylaniline
[0283]
[0284] Step 1) Preparation of 1-cyclopropyl-4-nitrobenzene
[0285]
[0286] 3.0 g (14.9 mmol) of 4-bromonitrobenzene and 1.7 g (19.4 mmol) of cyclopropylboronic acid were dissolved in 40 ml of toluene and 4 ml of water, respectively. To this was added 133 mg (0.6 mmol) of palladium acetate (Pd(OAc) 2 ), 418 mg (1.49 mmol) tricyclohexylphosphine (P(C 6 h 11 ) 3 ) and 6.8 g (49.2 mmol) of potassium carbonate (K 2 CO 3 ). It was purged with argon for 30 minutes, heated to a temperature in the range of 100°C to 110°C, and reacted for three hours. The reaction was identified as complete by TLC monitoring and celite filtration was performed under reduced pressure. Then, the filtered organic layer was concentrated under reduced pressure. The residue obtained by concentration was purified by column chromatography (ethyl acetate:hexane=1:9 (v / v)), whereby 2.1 g of the desired compound were obt...
preparation example 2
[0292] Preparation Example 2: Preparation of 2,6-difluoro-4-(pyrrolidin-1-yl)aniline
[0293]
[0294] At room temperature, 7.70 g (24.99 mmol) of tert-butyl (4-bromo-2,6-difluorophenyl) carbamate, 8.3 ml (99.96 mmol) of pyrrolidine, 561 mg (2.50 mol) of palladium acetate, 2.89 g (5.00 mmol) of 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene and 15.29 g (49.98 mmol) of cesium carbonate dissolved in 77 ml of toluene . Then, it was purged with argon for 5 minutes, heated to a temperature of 90° C., and reacted for 12 hours. The reaction was identified as complete by TLC monitoring and celite filtration was performed under reduced pressure. Then, the filtered organic layer was concentrated under reduced pressure. The residue obtained by concentration was purified by column chromatography, whereby 4.56 g of the desired compound were obtained (yield: 61%).
[0295] 1 H-NMR (300MHz, CDCl 3 ):δ6.05(d,2H),5.63(br,1H),3.21(m,4H),2.00(m,4H),1.48(s,9H)
[0296]
[0297] 4.56...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com