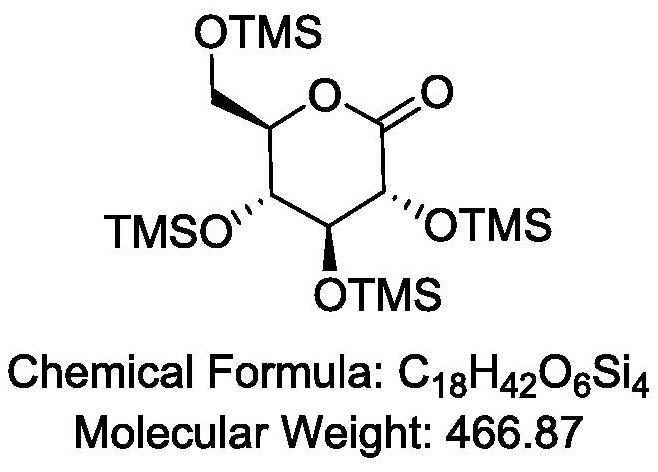
Method for preparing 2, 3, 4, 6-tetra-O-trimethylsilyl-D-glucolactone by using continuous flow micro-channel reactor
A technology of gluconolactone and microchannel reactor, which is applied in chemical instruments and methods, compounds of group 4/14 elements of the periodic table, organic chemistry, etc., and can solve the problems of low reaction efficiency, complex operation, and non-continuous production, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
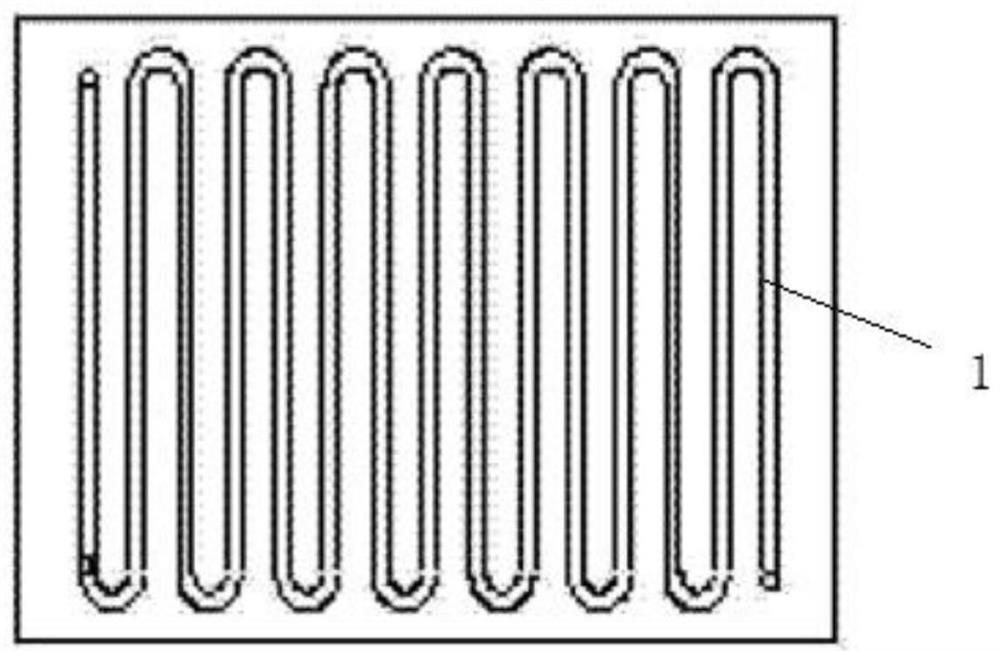
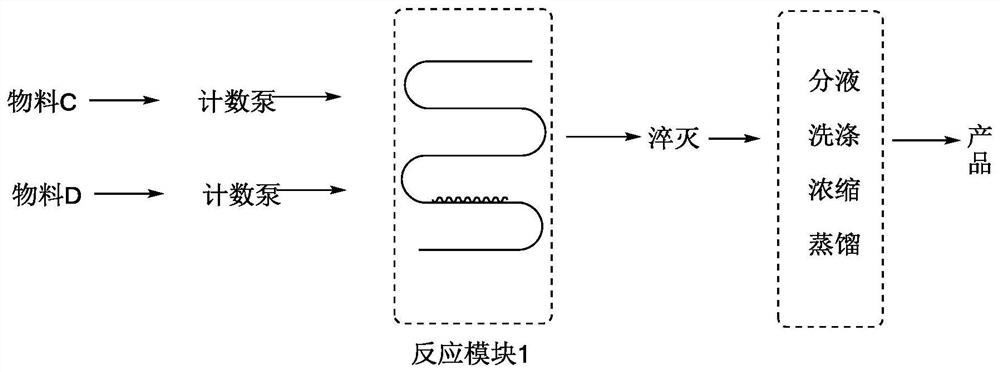
[0048] combine Figure 1-2 As shown, using microchannel reactor 1 (see figure 1 ), the preparation of 2,3,4,6-tetra-O-trimethylsilyl-D-gluconic acid by continuous flow microchannel reaction using hexamethyldisilazane to protect the hydroxyl group of 1,5-gluconolactone The method of lactone is carried out according to the following steps:
[0049] 1) Add 100g of 1,5-gluconolactone into 500ml of chloroform, stir and cool down to 5-10°C, keep it warm to obtain A test solution, about 600ml.
[0050] 2) Dissolve 2.8g of iodine in 10ml of chloroform to obtain B test solution, about 11ml.
[0051] 3) Add test solution B to test solution A dropwise at 5-10°C, and mix to obtain test solution C, about 610ml.
[0052]4) Dilute 361g of hexamethyldisilazane (4 equivalents) with 800ml of chloroform to obtain D test solution, about 1000ml.
[0053] 5) Stir 1000ml of chloroform at the outlet of the microchannel reactor, and control the temperature at 30-35°C.
[0054] 6) Configure 10 g o...
Embodiment 2
[0061] In Example 1, 271g of hexamethyldisilazane (3 equivalents) was added instead; other process conditions remained unchanged.
[0062] Subsequent operations such as dilution, quenching, pickling, pH adjustment, concentration, and distillation yielded 2,3,4,6-tetra-O-trimethylsilyl-D-gluconolactone with a product yield of 80%. 98% purity.
Embodiment 3
[0064] In Example 1, 452g hexamethyldisilazane (5 equivalents) was added instead; other process conditions were unchanged.
[0065] Subsequent operations such as dilution, quenching, pickling, pH adjustment, concentration, and distillation yielded 2,3,4,6-tetra-O-trimethylsilyl-D-gluconolactone with a product yield of 85%. 98.8% purity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com