NMDAR recombinant protein related to autoimmune encephalitis as well as coding sequence, preparation method and application of NMDAR recombinant protein
An autoimmune and recombinant protein technology, applied in the field of NMDAR recombinant protein, can solve the problems of difficulty in automation, high cost, and limited detection range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] This embodiment discloses the preparation method of the NMDAR recombinant protein of the present invention, specifically:
[0034] 1. Optimizing the nucleotide sequence encoding the recombinant protein
[0035] In order to increase the expression of recombinant protein in Escherichia coli, under the premise that the amino acid sequence of the recombinant protein remains unchanged, the nucleotide sequence encoding the recombinant protein is converted into the corresponding nucleotide sequence according to the preferred codons of Escherichia coli, and the optimized The sequence shown in SEQ ID NO.9. The CAI value of the nucleotide sequence before optimization was 0.84, and the CAI value of the optimized nucleotide sequence was 0.94. The results are attached Figure 5 shown.
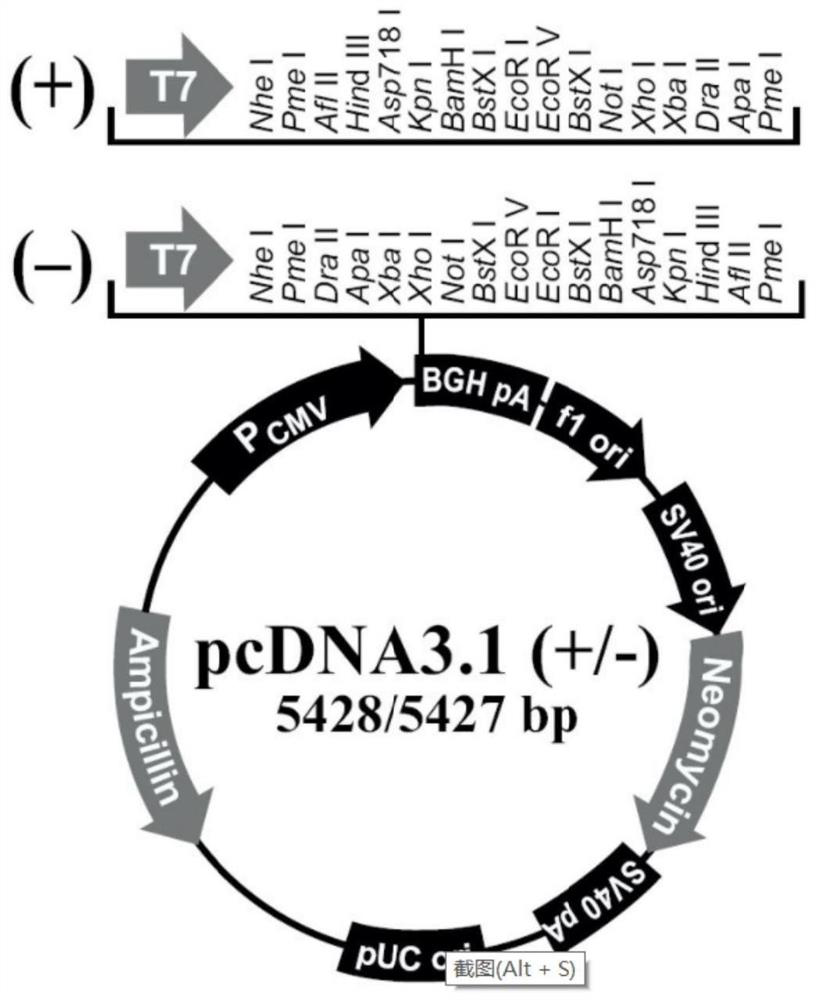
[0036] 2. Use the gene sequence shown in SEQ ID NO.9 after codon preference optimization, connect the signal peptide, His tag and KozAk sequence, and synthesize the target gene sequence shown in S...
Embodiment 2
[0066] This example discloses the use of the NMDAR recombinant protein prepared in Example 1 to prepare a quantitative detection kit for anti-NMDAR antibodies in human blood (magnetic particle chemiluminescence method) and to detect the immunoreactivity of the antigen.
[0067] Coupling NMDAR recombinant protein with magnetic particles, the procedure is to take 2mL of Tosyl magnetic bead stock solution, wash with coating buffer three times, then add coating buffer 10mL, NMDAR recombinant protein 500μL, 3M ammonium sulfate 5.25mL, at 37℃ Reaction 16h. After the reaction, the magnetic beads were taken out, the supernatant was removed, and 15.75 mL of blocking solution was added to react at 37°C for 9 hours. Finally, wash twice with blocking solution, wash once with magnetic bead protection solution, and then dilute to volume with magnetic bead protection solution.
[0068] Use NMDAR antibody to dilute the small sample according to the ratio of 1 / 100, 1 / 400, 1 / 1600, and 1 / 32000,...
Embodiment 3
[0074] According to the method of Example 2, the NMDAR recombinant protein was coupled with magnetic particles to prepare a quantitative detection kit for anti-NMDAR antibodies in human blood (magnetic particle chemiluminescence method), and the performance of the reagent was evaluated to verify the minimum detection limit of the detection reagent. , linearity and precision.
[0075] 1. Minimum detection limit verification
[0076] Using the zero concentration calibrator and the adjacent concentration calibrator, the minimum detection limit was back-calculated according to the calculation software, and the results are shown in the following table:
[0077] Table 2
[0078]
[0079] The measurement results showed that the minimum detection limit was 0.0295RU / mL, which was in line with the industry regulation not higher than 0.50RU / mL.
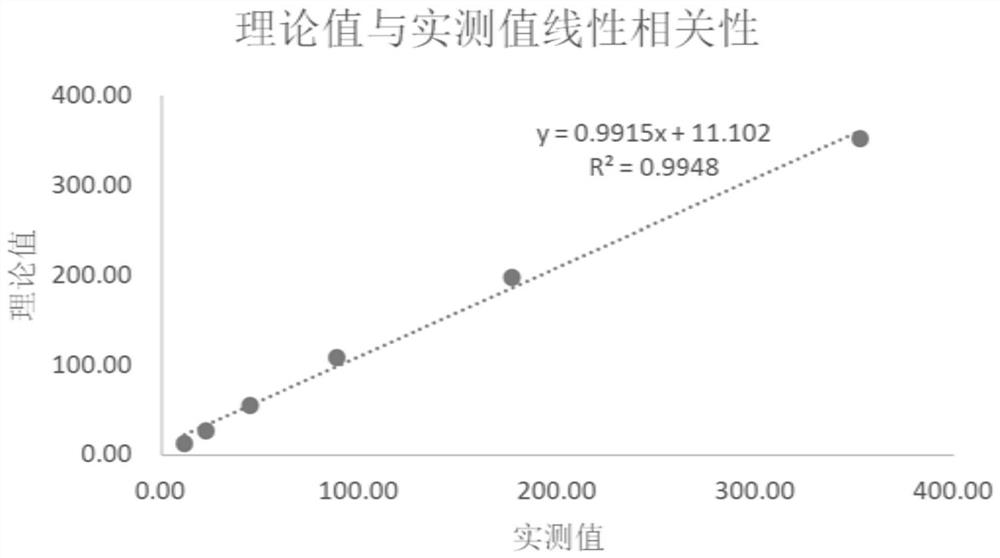
[0080] 2. Linearity Verification
[0081] Use clinical high-value samples to gradiently dilute, verify the linear correlation, and calcul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com