Novel compound preparation and application thereof in bacterial diseases
A compound preparation and bacterial technology, which is applied to a new compound preparation and its application in bacterial diseases, can solve the problems that the compatibility of bacteriophage and probiotics is difficult to achieve the expectation of comprehensive prevention and control of bacterial diseases.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
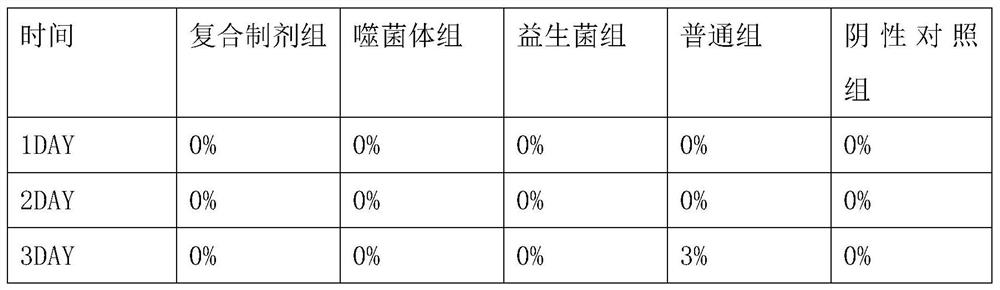
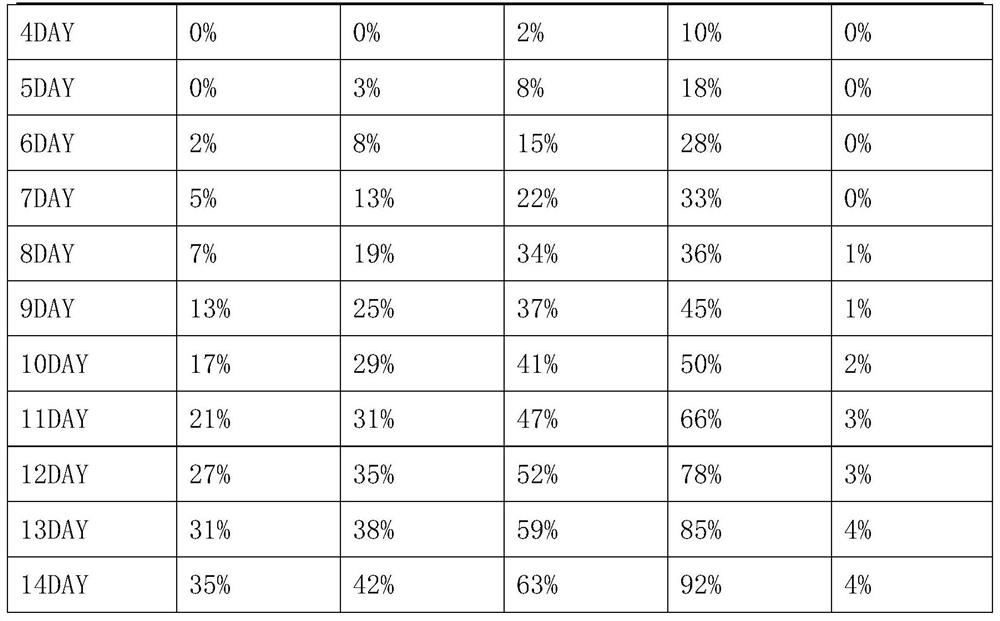
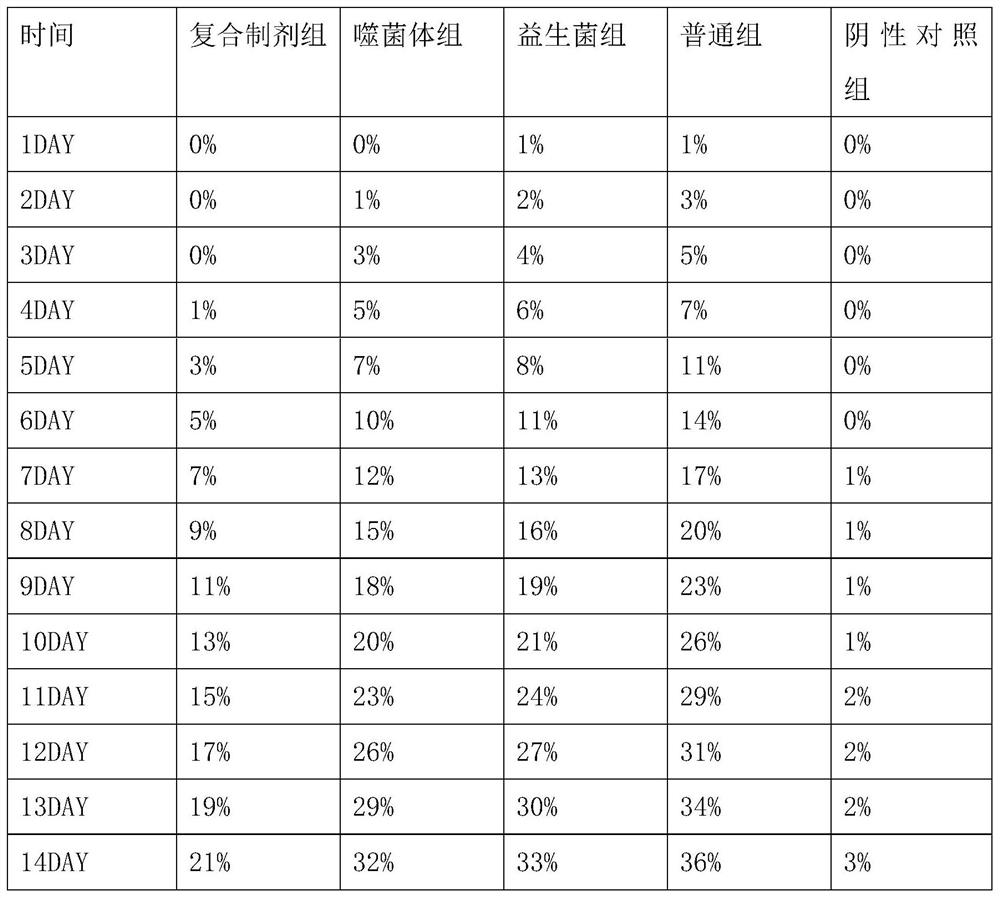
Examples
Embodiment 1
[0040] Preparation of Vibrio parahaemolyticus Phage
[0041] Seed culture: Inoculate the activated Vibrio parahaemolyticus host bacteria into TSB medium with an inoculation ratio of 2%, 220 rpm, and culture at 37° C. for 4 hours to obtain the seed liquid of the Vibrio parahaemolyticus host bacteria.
[0042] Enrichment culture: insert Vibrio parahaemolyticus phage and host bacteria into TSB medium, and the MOI value is 5×10 -6 , the host bacteria inoculation ratio is 5%, 190 rpm, 37 ° C for 5 hours.
[0043] Sterilization and purification: After the fermentation, the fermented liquid was centrifuged at 7000rpm for 10 minutes to collect the supernatant, and the supernatant was sterilized and purified with a 0.22 μm filter membrane to obtain Vibrio parahaemolyticus phage water. The titers of VP46, VP48 and VP7 were respectively 5.7×10 10 PFU / mL, 4.8×10 10 PFU / mL and 4.5×10 10 PFU / mL.
[0044] Drying: add protective agent according to the ratio of 3:1, the ratio of protectiv...
Embodiment 2
[0046] Preparation of Vibrio alginolyticus Phage
[0047] Seed cultivation: Inoculate the activated Vibrio alginolyticus host bacteria into TSB medium with an inoculation ratio of 2%, 220 rpm, and culture at 37° C. for 4 hours to obtain a seed liquid of the Vibrio alginolyticus host bacteria.
[0048] Enrichment culture: insert Vibrio alginolyticus phage and host bacteria into TSB medium, and the MOI value is 5×10 -6 , the host bacteria inoculation ratio is 5%, 190 rpm, 37 ° C for 5 hours.
[0049] Sterilization and purification: After the fermentation, the fermented liquid was centrifuged at 7000rpm for 10 minutes to collect the supernatant, and the supernatant was sterilized and purified with a 0.22 μm filter membrane to obtain Vibrio alginolyticus phage water, the titers of VAP7, VAP9 and VAP21 were 3.7 ×10 10 PFU / mL, 2.8×10 10 PFU / mL and 2.5×10 10 PFU / mL.
[0050] Drying: add protective agent according to the ratio of 3:1, the ratio of protective agent is: 15 parts of...
Embodiment 3
[0052] Preparation of coliphage
[0053] Seed cultivation: Inoculate the activated E. coli host bacteria into TSB medium with an inoculation ratio of 2%, culture at 220 rpm, and 37° C. for 4 hours to obtain E. coli host bacteria seed liquid.
[0054] Enrichment culture: insert Vibrio parahaemolyticus phage and host bacteria into TSB medium, and the MOI value is 5×10 -6 , the host bacteria inoculation ratio is 5%, 190 rpm, 37 ° C for 5 hours.
[0055] Sterilization and purification: After the fermentation, the fermented liquid was centrifuged at 7000rpm for 10 minutes to collect the supernatant, and the supernatant was sterilized and purified with a 0.22 μm filter membrane to obtain E. coli phage water. 10 PFU / mL, 3.4×10 10 PFU / mL and 3.7×10 10 PFU / mL.
[0056] Drying: add protective agent according to the ratio of 3:1, the ratio of protective agent is: 15 parts of skimmed milk powder, 3 parts of glycerin, 4 parts of sodium glutamate, 5 parts of sucrose, mix and dry, crush ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com