Synthesis and refining method of chlorpheniramine maleate intermediate
A kind of technology of chlorpheniramine acid intermediate and refining method is applied in the field of synthesis and purification of the production of chlorpheniramine maleate intermediate, which can solve the problem of low yield, dark color of chlorpheniramine maleate and impurity High content and other problems, to achieve high yield, mild reaction conditions, simple post-treatment effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
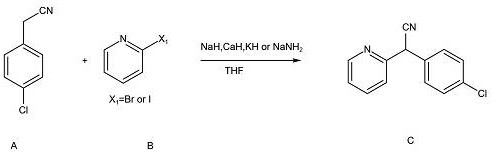
[0023] Put 60% NaH (7.2g, 0.18mol) into THF (100ml), lower the temperature to 2°C, and add p-chlorophenylacetonitrile (22.7g, 0.15mol) in THF (20ml) dropwise under temperature control, During the dropwise addition, the temperature was raised slowly. After the dropwise addition, stir at room temperature for 1 hour. After the timing, the temperature was raised to 40°C. The heating was removed, and 2-bromopyridine (15.8g, 0.1mol) was slowly added dropwise. The temperature rose violently during the dropwise addition. After the dropwise addition, the temperature dropped to At about 40°C, start heating to reflux, time the reaction for about 10 hours (TLC detects that the raw material disappears), cool down to room temperature, quench the reaction with water, extract with toluene, and concentrate the organic phase under reduced pressure until no liquid drops out to obtain the crude product. After the temperature is below 30°C, add 126.4g of n-hexane / ethyl acetate (7:1) mixed solvent ...
Embodiment 2
[0025] Put 95% CaH (8.84g, 0.2mol) into THF (100ml), lower the temperature to 2°C, and add p-chlorophenylacetonitrile (22.7g, 0.15mol) in THF (20ml) dropwise, During the dropwise addition, the temperature was raised slowly. After the dropwise addition, stir at room temperature for 1 hour. After the timing, the temperature was raised to 40°C. The heating was removed, and 2-bromopyridine (15.8g, 0.1mol) was slowly added dropwise. The temperature rose violently during the dropwise addition. After the dropwise addition, the temperature dropped to At about 40°C, start heating to reflux, time the reaction for about 10 hours (TLC detects that the raw material disappears), cool down to room temperature, quench the reaction with water, extract with toluene, and concentrate the organic phase under reduced pressure until no liquid drops out to obtain the crude product. After the temperature is below 30°C, add 126.4g of n-hexane / ethyl acetate (7:1) mixed solvent to the crude product for s...
Embodiment 3
[0027] Put 60% NaH (7.2g, 0.18mol) into THF (100ml), lower the temperature to 2°C, and add p-chlorophenylacetonitrile (22.7g, 0.15mol) in THF (20ml) dropwise under temperature control, During the dropwise addition, the temperature was raised slowly. After the dropwise addition, stir at room temperature for 1 hour. After the timing, the temperature was raised to 40°C. The heating was removed, and 2-bromopyridine (15.8g, 0.1mol) was slowly added dropwise. The temperature rose violently during the dropwise addition. After the dropwise addition, the temperature dropped to At about 40°C, start heating to reflux, time the reaction for about 10 hours (TLC detects that the raw material disappears), cool down to room temperature, quench the reaction with water, extract with toluene, and concentrate the organic phase under reduced pressure until no liquid drops out to obtain the crude product. After the temperature is below 30°C, add 126.4g of petroleum ether / ethyl acetate (7:1) mixed s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com