Dydrogesterone synthesis methodand compound
A technology of chemical reaction and photochemical reaction, applied in the direction of steroids, organic chemistry, etc., can solve the problems of increased product impurities, low yield of target products, low photochemical yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
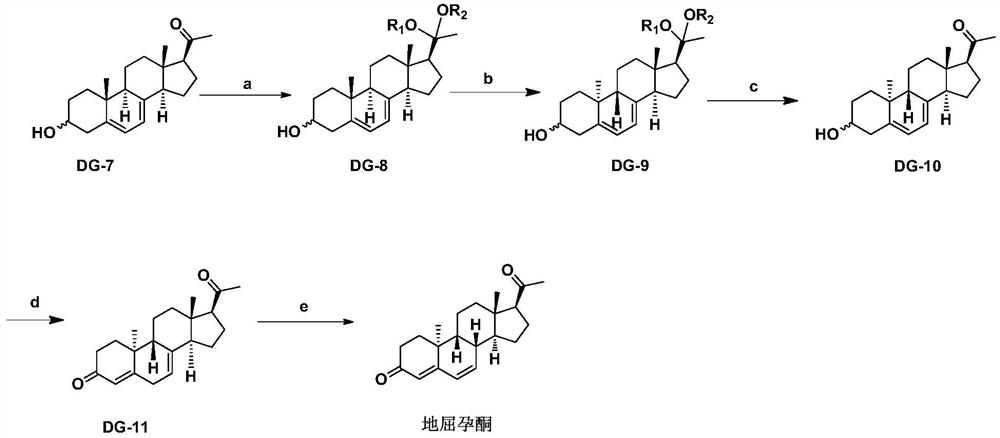
[0036] Add 500g DG-7, 500g ethylene glycol, 500g triethyl orthoformate, 5g p-toluenesulfonic acid and 5000ml dichloromethane into the reaction flask, stir at room temperature and complete the reaction, add 1000ml water and stir to separate the liquid, organic The phase was concentrated under reduced pressure to obtain solid DG-8.1 with a yield of 95.2%.
[0037] ESI-HRMS theoretical value: C 23 h 34 o 3 [M+H] + 359.2508, found 359.2510.
[0038] 1H-NMR (δ, ppm, CDCl 3 ):5.70-5.55(m,1H);5.39-5.38(m,1H);4.02-3.84(m,4H);3.66-3.59(m,1H);2.48-2.43(m,1H);2.30-2.24 (m,1H);2.16-2.12(m,1H);1.98-1.94(m,1H);1.89-1.87(m,4H);1.83-1.66(m,4H);1.61-1.53(m,1H) ;1.51-1.41(m,2H);1.32-1.22(m,5H);0.94(s,3H);0.71(s,3H).
Embodiment 2
[0040]
[0041] Add 500g DG-7, 114g triethyl orthoformate, 1.4g p-toluenesulfonic acid and 540ml methanol into the reaction flask, react at 50℃~60℃ for three hours, reduce pressure, add 500mL ethyl acetate to extract, then use 500mL Washed with saturated sodium bicarbonate, washed with 500 mL saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated to obtain DG-8.2 with a yield of 93.7%.
[0042] ESI-HRMS theoretical value: C 23 h 36 o 3 [M+H] + 361.2664, found 361.2669.
[0043] 1H-NMR (δ, ppm, CDCl 3):5.74-5.58(m,1H); 5.40-5.37(m,1H); 4.05(s,3H); 3.84(s,3H); 3.70-2.45(m,2H); );1.90-1.86(m,4H);1.72-1.67(m,4H);1.60-1.43(m,3H);1.35-1.19(m,5H);0.89(s,3H);0.74(s,3H ).
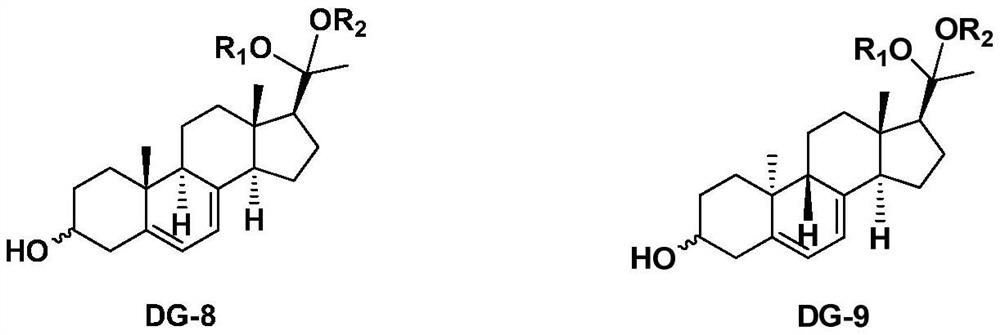
[0044] Synthesis of Compound DG-9 Series
Embodiment 3
[0046]
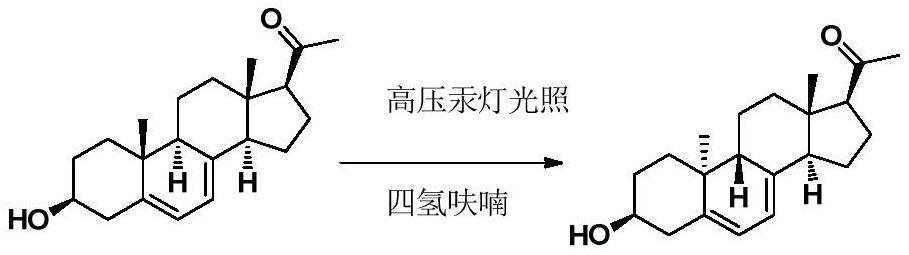
[0047] Add 100g of DG-8.1, 100g of triethylamine into 10L of absolute ethanol and stir to dissolve, then add 0.5g of 2,6-di-tert-butyl-p-cresol, add an internal light with quartz cold hydrazine (transmitting wavelength 200-300nm) photochemical reactor. Turn on the stirring, control the temperature at 10-15°C to circulate the freezing liquid, and turn on the 500W high-pressure mercury lamp to irradiate for 8 hours. Turn off the high-pressure mercury lamp, filter the reaction solution, collect the filtrate, add the filtrate to the internally illuminated photochemical reactor with high borosilicate glass cold hydrazine (transmissible wavelength 310nm-400nm), turn on the stirring, and turn on the 500W high-pressure mercury lamp After irradiating for 16 hours, turn off the high-pressure mercury lamp, concentrate the reaction solution under reduced pressure at 35°C, freeze and crystallize to obtain DG-9.1 as a white solid with a purity of 99.3% and a yield of 70.6%.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com