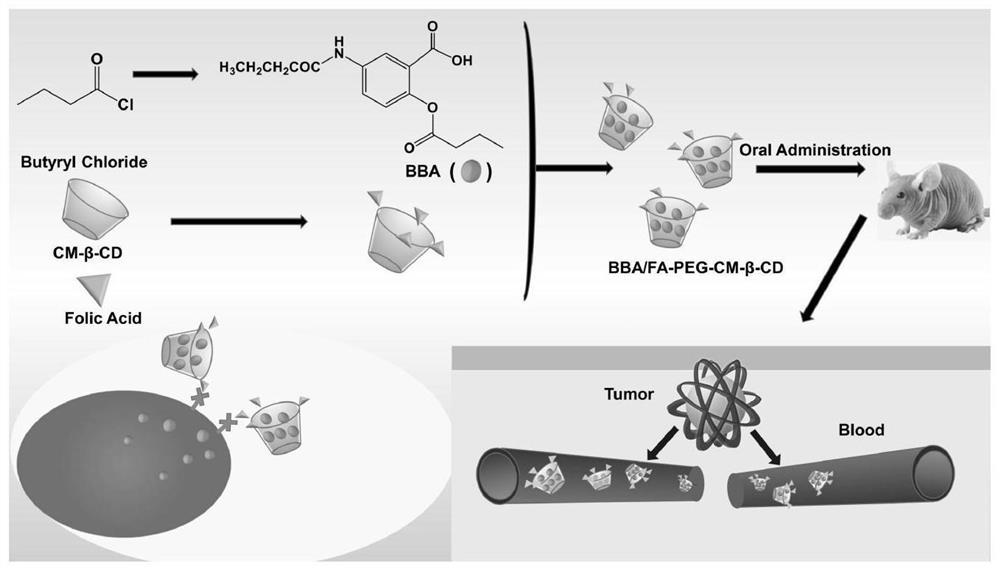
FA-mediated BBA/CM-beta-CD targeted drug delivery system as well as preparation method and application thereof
A FA-PEG-CM-, drug delivery system technology, applied in the field of medicine, can solve the problems of limiting the maximum allowable dose, drug concentration is not enough to achieve the effective concentration of tumor treatment, etc., to achieve the effect of enhancing drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
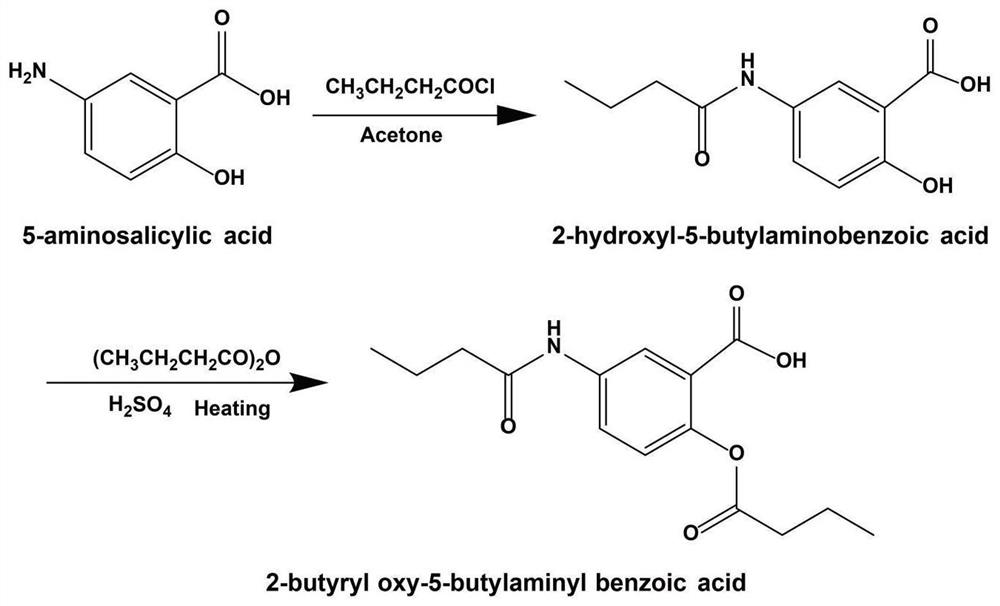
[0039] Example 1. Preparation of 2-butyryloxy-5-butyrylaminobenzoic acid (BBA)
[0040] 1. Synthesis of 2-hydroxy-5-butanamide benzoic acid
[0041] Weigh 0.4g (1.31mmol) of 5-aminosalicylic acid and place it in a 50mL single-neck round-bottomed flask, add 16mL of saturated sodium bicarbonate solution, stir and dissolve, and slowly add 0.27 g of butyryl chloride to the round-bottomed flask under an ice bath. 8 mL of acetone solution. After the dropwise addition was completed, the mixture was stirred magnetically and reacted at room temperature for 4h. TLC detected that the reaction was complete, the solvent was removed by rotary evaporation under reduced pressure, and the remaining liquid was cooled and acidified by adding 0.5 mL of hydrochloric acid. The solution was turbid, filtered with suction to obtain a light chocolate-colored precipitate, and finally dried in vacuum for 12 hours.
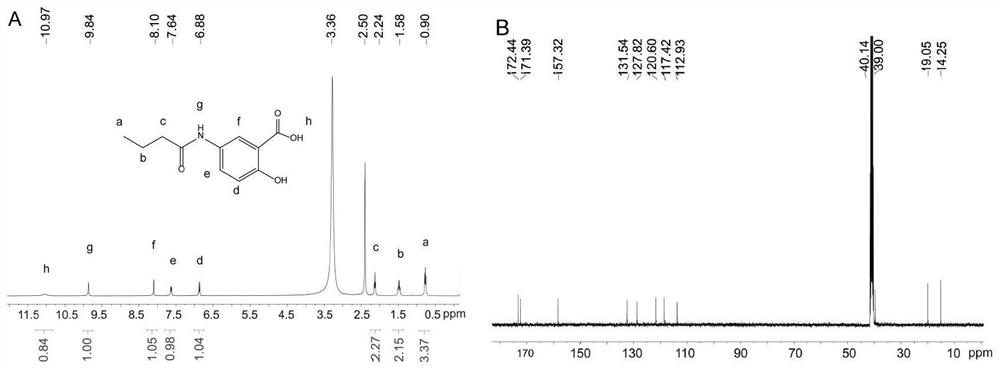
[0042] like image 3 shown, the 2-hydroxy-5-butyramide benzoic acid samples 1 H NMR a...
Embodiment 2
[0049] Example 2. Preparation of FA-modified 2-butyryloxy-5-butyrylaminobenzoic acid / carboxymethyl-β-cyclodextrin inclusion complex (BBA / FA-PEG-CM-β-CD) and Characterization
[0050]BBA / CM-β-CD inclusion complex has a good sustained release effect, which can improve drug efficacy and reduce toxic and side effects; however, it is difficult for BBA / CM-β-CD to pass through the plasma membrane of tumor cells, resulting in a low rate of uptake by target cells. . Therefore, in the present application, FA was selected to modify BBA / CM-β-CD to further increase the uptake rate of BBA / CM-β-CD by tumor cells.
[0051] The FA-mediated targeted drug delivery system can actively target the drug to the target organ or target cell, and realize the active targeted transportation of the folic acid-drug carrier conjugate, thereby improving the targeting of the drug and reducing the toxic and side effects of the drug. Compared with other ligands, FA has the advantages of high affinity to recept...
Embodiment 3
[0095] Example 3 In vitro study of inclusion compound
[0096] 1. Cell proliferation inhibition assay
[0097] (1) In this example, CaCo-2 cells (human cloned colon adenocarcinoma cells) and SW620 cells (human colon cancer cells) were used as model cells, and the MTT method was used to evaluate 5-ASA, NaB, 5-FU, BBA, FA- In vitro anticancer effects of PEG-CM-β-CD, BBA / CM-β-CD and BBA / FA-PEG-CM-β-CD. Take the cells in logarithmic growth phase and prepare a single cell suspension with 10% Gibco fetal bovine serum (FBS) and 1% penicillin-streptomycin in DMEM medium, and the cell concentration is 3.5×10 4 cells / mL, inoculate 100 μL of suspension in each well of a 96-well plate, set a blank cell control well, in 5% CO 2 Incubator overnight. Add PBS to the periphery of the 96-well plate to prevent excessive liquid evaporation during incubation.
[0098] (2) Discard the original medium, add different concentrations of BBA, FA-PEG-CM-β-CD, BBA / CM-β-CD, BBA / FA-PEG-CM-β-CD to the cu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com