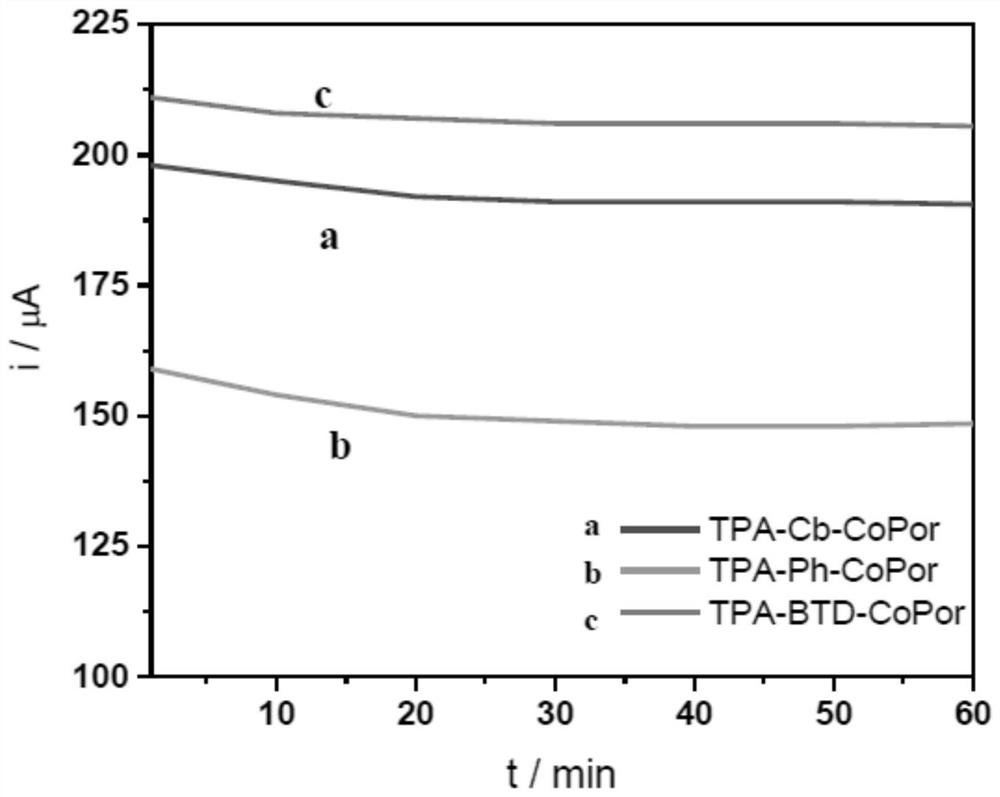
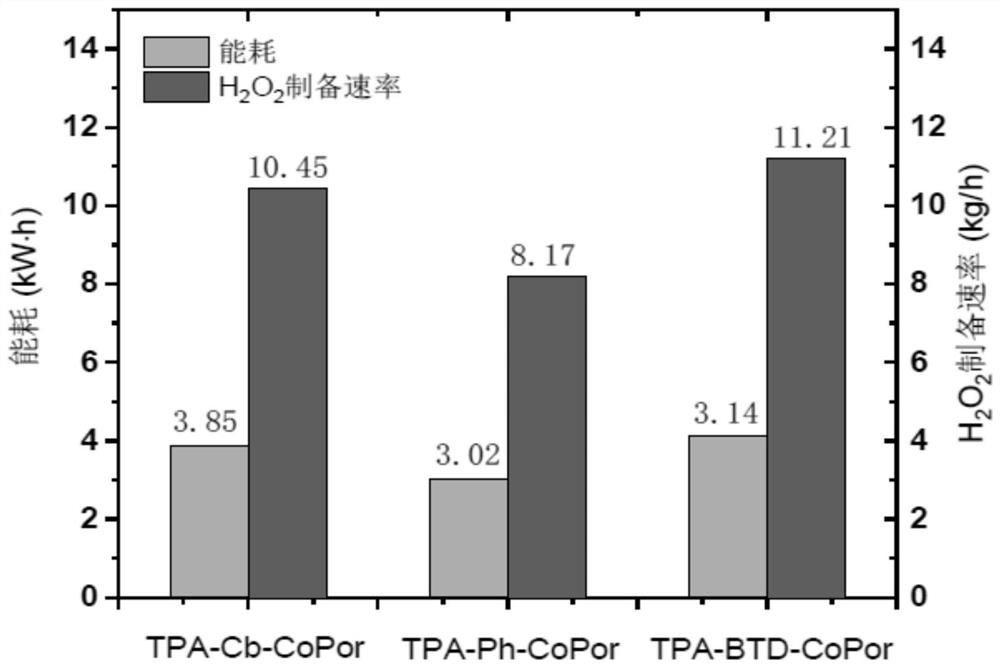
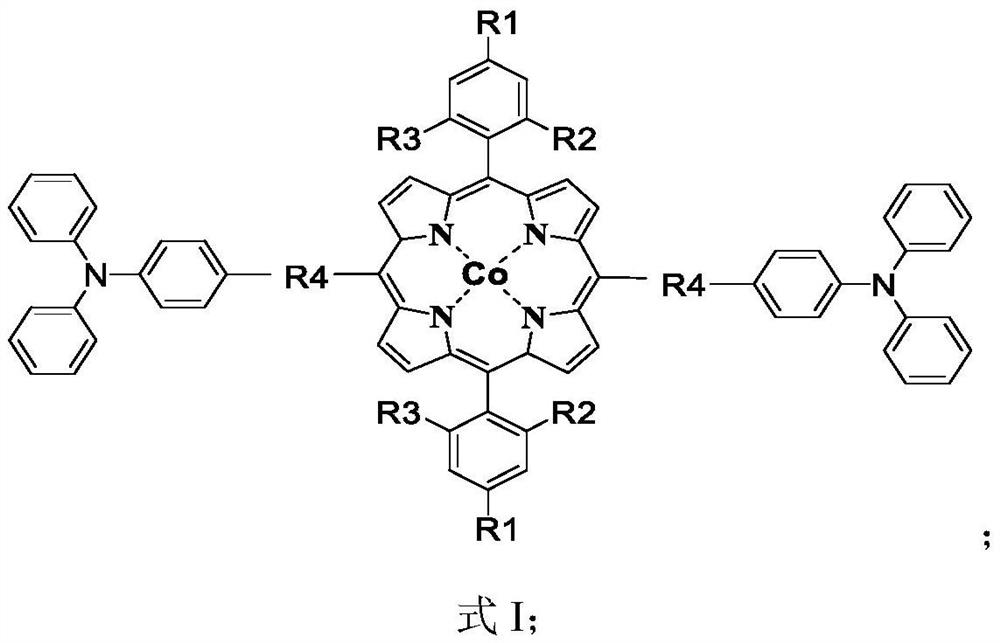
A kind of triphenylamine-based cobalt porphyrin catalyst and its preparation method and application
A technology of triphenylamine-based cobalt porphyrins and catalysts, which is applied in the field of electrocatalytic oxygen reduction to produce hydrogen peroxide, can solve the problems of changing the proton concentration of the catalytic interface, increasing the explosion risk, and the influence of oxygen adsorption, and achieves good electrocatalysis. Oxygen reduction activity, improvement of hydrogen peroxide selectivity, effect of high hydrogen peroxide selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] (1) Synthesis of the first intermediate 6-(4-(diphenylamino)phenyl)-9-ethylcarbazole-3-carbaldehyde (Suzuki coupling reaction):
[0063] 1.5mmol (455mg) of 6-bromo-9-ethyl-9H-carbazole-3-carbaldehyde and 2mmol (579mg) of 4-(diphenylamino)phenylboronic acid were dissolved in 35mL of toluene, 5mL of sodium carbonate solution (2M) was added, After stirring for 1 minute, 200 mg of tetrakis(triphenylphosphine)palladium(0) was added. The system was refluxed at 95°C for 45 hours under nitrogen protection. Then the mixed solution was washed with saturated salt solution, acid washed with dilute hydrochloric acid, and extracted with dichloromethane. The organic phase was separated, dried over anhydrous sodium sulfate and spun dry, and the crude product was purified by silica gel column chromatography with dichloromethane / n-hexane (2:1) as eluent. 608.9 mg of yellow solid product was obtained in 87% yield.
[0064] (2) Synthesis (condensation reaction) of the second intermediat...
Embodiment 2
[0076] (1) Synthesis of the first intermediate: 4-(4-(diphenylamino)phenyl)-benzaldehyde (Suzuki coupling reaction): the synthesis method is the same as that of step (1) in Example 1, and the yield is 90%.
[0077] (2) The third intermediate: 5,15-bis(1-(4-(diphenylamino)phenyl)-(phenyl-4-yl))-10,20-bis(2,4,6- Synthesis (condensation reaction) of trimethylphenyl) porphyrin: the synthesis method is the same as the step (3) of Example 1. Yield 8.0%, purple solid.
[0078] (3) Compound TPA-Ph-CoPor: 5,15-bis(1-(4-(diphenylamino)phenyl)-(phenyl-4-yl))-10,20-bis(2,4, Synthesis of 6-trimethylphenyl)cobalt(II) porphyrin (coordination reaction): the synthesis method is the same as that of the compound TPA-Cb-CoPor in Example 1, the yield is 94.0%, and it is a dark red solid.
[0079] The specific process is as follows:
[0080]
[0081] Preparation of carbon material-supported triphenylamine-based cobalt porphyrin composite catalyst: Disperse a mixture of 3 mg of compound TPA-Ph...
Embodiment 3
[0085] (1) The first intermediate: 7-(4-(diphenylamino)phenyl)benzo[c][1,2,5]thiadiazole-4-carbaldehyde (g): the synthesis method is the same as that in Example 1 of step (1) in 88% yield.
[0086] (2) The third intermediate: 5,15-bis(3-(4-(diphenylamino)phenyl)-(2,1,3-benzothiadiazol-4-yl))-10,20 -Two (2,4,6-trimethylphenyl) porphyrin (condensation reaction): the synthetic method is the same as the step (3) of embodiment 1. Yield 12.3%, purple solid.
[0087] (3) Compound TPA-BTD-CoPor: 5,15-bis(3-(4-(diphenylamino)phenyl)-(2,1,3-benzothiadiazol-4-yl))-10 , 20-bis(2,4,6-trimethylphenyl) cobalt (II) porphyrin synthesis (coordination reaction): the synthesis method is the same as the compound TPA-Cb-CoPor of Example 1, the yield is 89%, dark red solid,
[0088] The specific process is as follows:
[0089]
[0090] Preparation of carbon material-supported triphenylamine-based cobalt porphyrin composite catalyst: Disperse a mixture of 3 mg of compound TPA-BTD-CoPor and 10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com