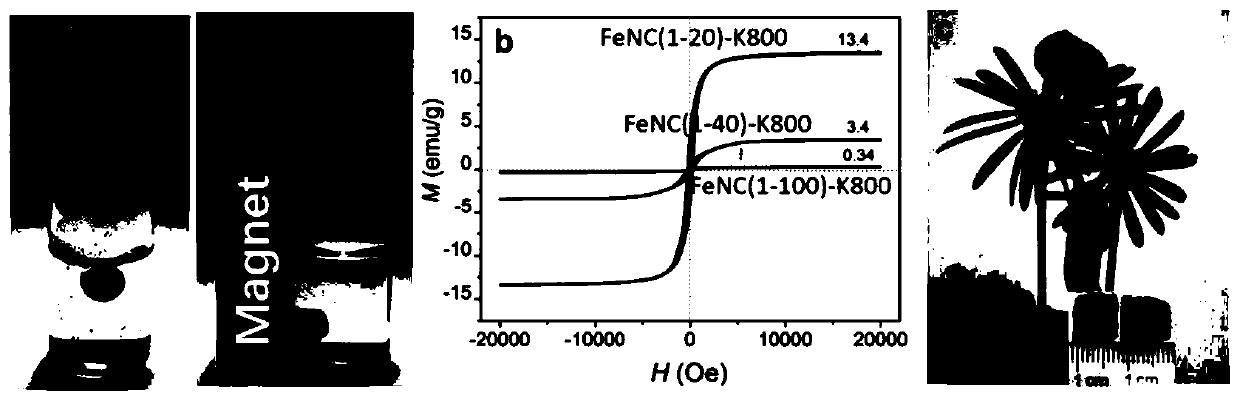
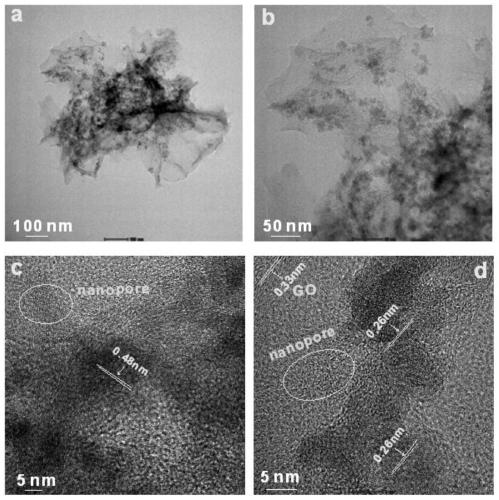
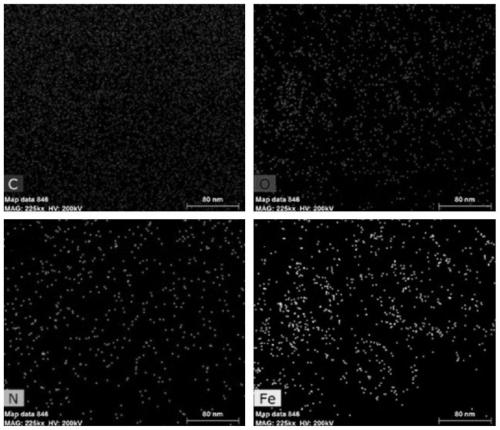
Preparation method of iron-nitrogen co-doped magnetic porous graphitized nano carbon aerogel
A technology of porous graphite and carbon aerogel, applied in nano-carbon, chemical instruments and methods, carbon compounds, etc., can solve the problems of long preparation cycle, high risk, complicated procedures, etc., achieve good electrocatalytic oxygen reduction activity, reduce burden effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A preparation method of iron-nitrogen co-doped magnetic porous graphitized nano-carbon aerogel, comprising the following steps:
[0027] 1) CS-Fe 3+ Preparation of chelate aerogel: Dissolve 3g chitosan (CS, deacetylation degree 88%, molecular weight about 600000) in 110mL 2wt% HAc solution, after complete dissolution, add 10mL dissolved FeCl with a certain mass 3 ·6H 2 O iron salt solution, mix well, continue to stir and react for half an hour, let stand for several hours to remove bubbles, obtain a uniform and stable red-brown viscous transparent solution, pour into a 24-well plate, freeze at -75 ℃ for 12 hours, and then freeze in vacuum Dry to remove moisture to obtain CS-Fe 3+ Chelate aerogel (CS-Fe 3+ aerogel). According to the residual mass of chitosan after calcination at 800 °C is about 36% of the initial mass, the addition of FeCl is calculated based on the ratio of the mass of Fe element added to the residual mass of chitosan. 3 ·6H 2 quality of O.
[00...
Embodiment 2
[0031] A preparation method of iron-nitrogen co-doped magnetic porous graphitized nano-carbon aerogel, comprising the following steps:
[0032] 1) CS-Fe 3+ Preparation of chelate aerogel: Dissolve 3g chitosan (CS, deacetylation degree 88%, molecular weight about 600000) in 3wt% HAc solution with a mass fraction of 3wt%, after complete dissolution, add 10mL to dissolve The iron salt solution with a certain quality of ferric nitrate, mix well, continue to stir and react for half an hour, and let stand for several hours to remove foam to obtain a homogeneous and stable red-brown viscous and transparent solution, pour it into a 24-well plate, and freeze at -75 ℃ for 10 hours Then, vacuum freeze-drying to remove moisture to obtain CS-Fe 3+ Chelate aerogel (CS-Fe 3+ aerogel). According to the residual mass of chitosan after calcination at 750℃ is about 36% of the initial mass, the mass of ferric nitrate added is calculated by the ratio of the mass of Fe element added to the resid...
Embodiment 3
[0036] A preparation method of iron-nitrogen co-doped magnetic porous graphitized nano-carbon aerogel, comprising the following steps:
[0037] 1) CS-Fe 3+ Preparation of chelate aerogel: Dissolve 3g chitosan (CS, deacetylation degree 88%, molecular weight about 600000) in a 4wt% HAc solution with a mass fraction of 4wt%, after complete dissolution, add 10mL to dissolve There is a certain quality of FeCl 3 ·6H 2 O iron salt solution, mix well, continue to stir and react for half an hour, let stand for several hours to remove bubbles, obtain a uniform and stable red-brown viscous transparent solution, pour it into a 24-well plate, freeze at -75 ℃ for 11 hours, and then freeze in vacuum Dry to remove moisture to obtain CS-Fe 3+ Chelate aerogel (CS-Fe 3+ aerogel). According to the residue mass of chitosan after calcination at 700℃ is about 36% of the initial mass, the addition of FeCl is calculated based on the ratio of the mass of Fe element added to the residual mass of ch...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electric potential / voltage | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com