Compound with EGFR and Wnt dual inhibition effects and preparation method and application thereof
A technology of inhibition and compounds, applied in organic chemistry, medical preparations containing active ingredients, drug combinations, etc., to achieve strong practical value, easy to achieve large-scale production, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the preparation of formula I compound (abbreviated as JK11):
[0041]
[0042] At room temperature, the compound of formula II (4-(4-amino-1H-pyrazol-1-yl)-1-tert-butoxycarbonyl-piperidine, 53.3mg, 0.2mmol) was dissolved in DMF (5ml), Then add potassium bicarbonate aqueous solution (3mol / L, 0.53ml, 1.6mmol) and FSO 2 N 3 (Fluorosulfonyl azide) in methyl tert-butyl ether solution (0.4mol / L, 2.2ml, 0.44mmol), mix the reaction system uniformly and then stir and react at room temperature for 1 hour;
[0043] End the reaction, add 0.25ml sodium ascorbate aqueous solution (0.5mol / L, 0.125mmol) to the reaction system to quench the reaction, to obtain a reaction solution containing the compound of formula III;
[0044] Then, the compound of formula IV (N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)-quinazolin-4-amine hydrochloride, 77.4 mg, was added to the reaction solution. 0.18mmol) and the catalyst formed by compounding 0.2ml CuSO4 aqueous solution (0.1mol...
Embodiment 2
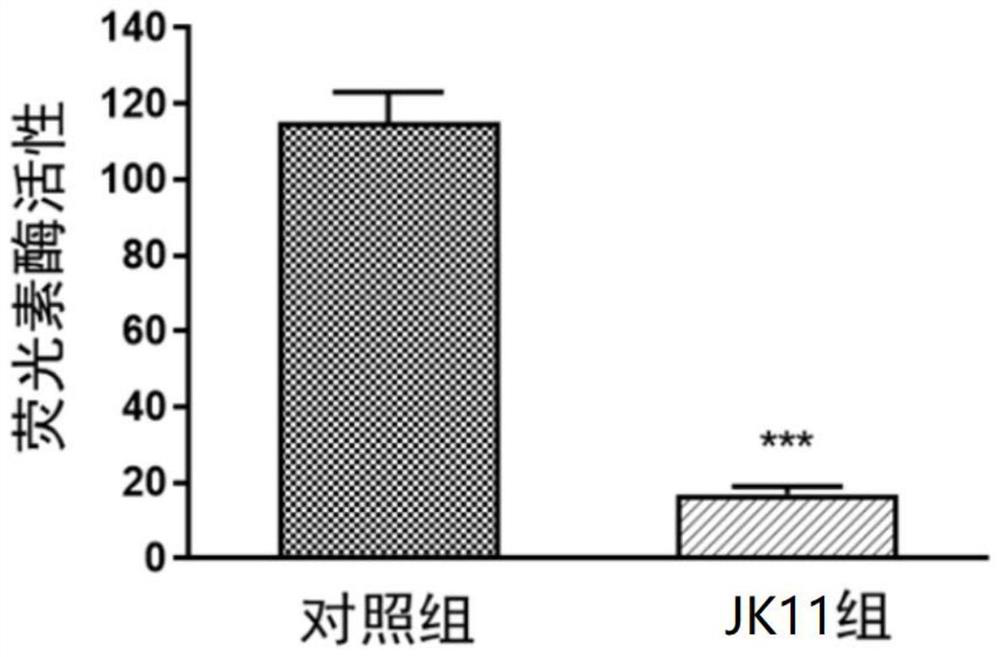
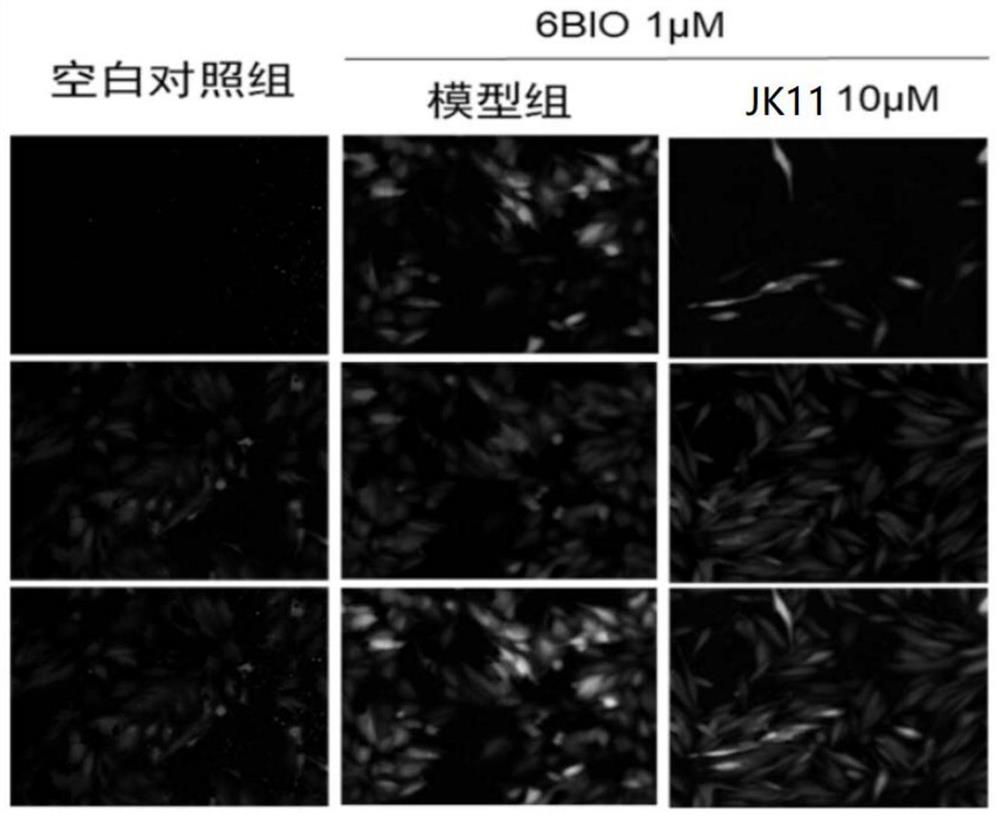
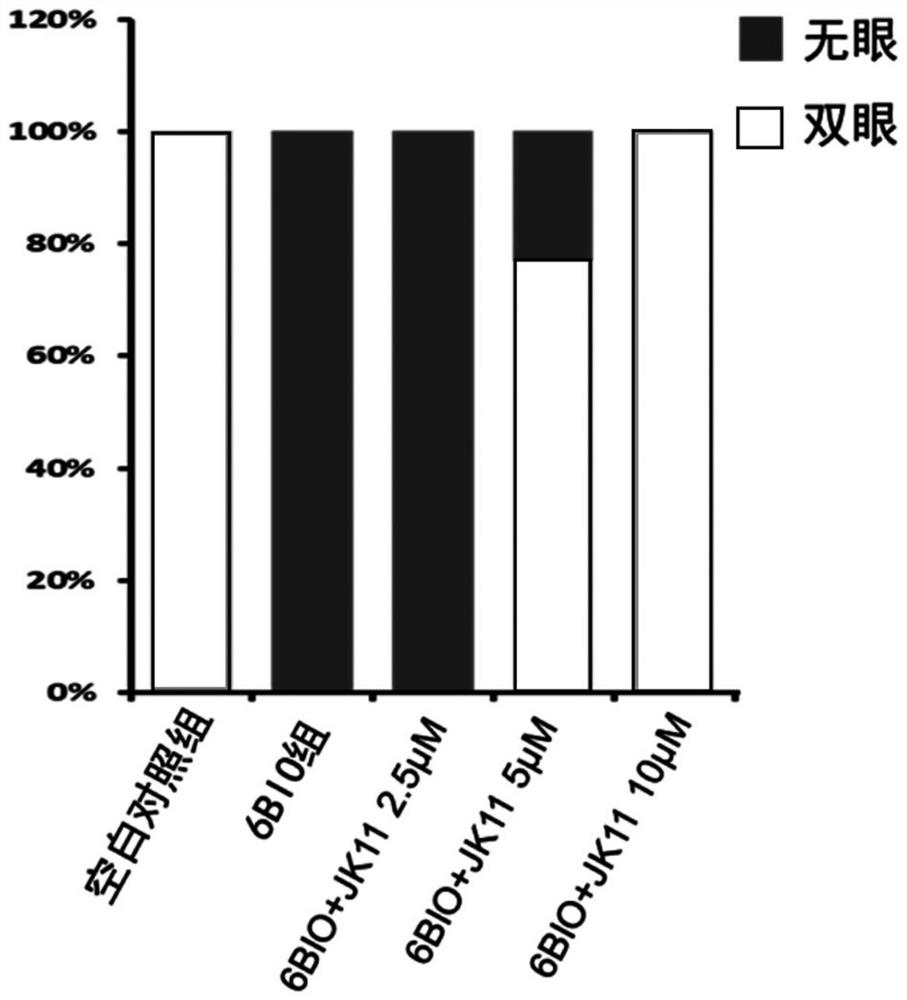
[0048] Embodiment 2: the inhibitory effect of formula I compound on Wnt / β-catenin pathway
[0049] Experimental materials: 293FT cells were purchased from the Cell Bank of the Chinese Academy of Sciences; EPT2 cells stably integrated with the TGC reporter system were from the University of Bergen, Norway; HCT116 cells were purchased from the Cell Bank of the Chinese Academy of Sciences; SW480 cells were purchased from ATCC; AB zebrafish were purchased from the National Zebra Fish Resource Center.
[0050] TOP / Flash experimental procedure: 293FT cells were inoculated into 96-well white plate (20,000 cells / well). After 24 hours, the plate was given transfection TOP / Flash plasmid and Renilla plasmid. After 6 hours, JK11 (10 μ M) was given. After 24 hours of treatment, the Luciferase activity.
[0051] EPT2-TGC cell verification steps: Inoculate EPT2 cells with a stably integrated TGC reporter system in a 96-well plate, and give 1 μM 6-bromoindirubin-3'-oxime (6-Bronoindirubin-3'...
Embodiment 3
[0057] Embodiment 3: the inhibitory effect of formula I compound on EGFR
[0058] Experimental materials: A549 cells were purchased from the Cell Bank of the Chinese Academy of Sciences; A549 cells were purchased from the Cell Bank of the Chinese Academy of Sciences; P-EGFR, EGFR and GAPDH antibodies were purchased from CST; the control drug Erlotinib was purchased from Sigma.
[0059] EGFR kinase detection: EGFR kinase inhibitory activity was tested by LANCE Ultra enzyme activity evaluation method. Add 2.5 μL kinase solution to each well according to the arrangement, add 2.5 μL reaction solution to the control well, add 2.5 μL compound (JK11 or positive control)) solution to each well according to the arrangement, add 2.5 μL reaction solution to the control well, add Add 10 μL of Eu-anti-phospho-4E-BP1 and EDTA reaction reagent to 5 μL of substrate solution, centrifuge for mixing, and place it at room temperature for 60 minutes. The final concentration of Eu-anti-phospho-4E-B...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com