Method for continuously preparing 2-benzyl isoindolinone compound by adopting microchannel reactor
A technology of benzyl isoindolinone and micro-channel reactor, which is applied in directions such as organic chemistry, fermentation, etc., can solve the problems of hidden dangers of safety and environment, high cost of industrial scale-up production, limited practicability and the like, and achieves low production cost, The effect of good product quality and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
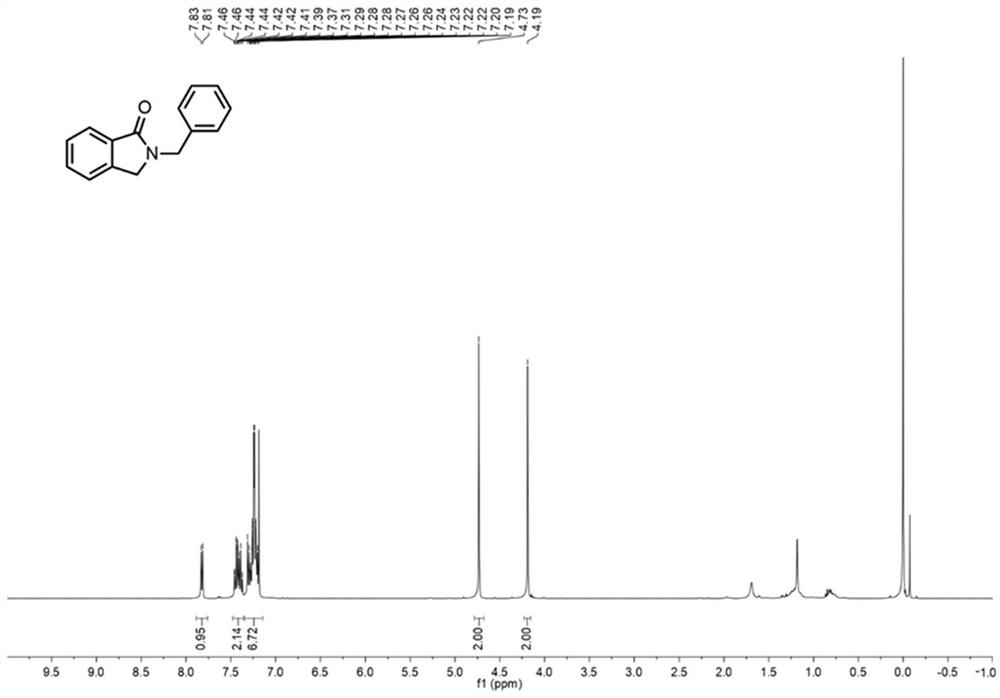
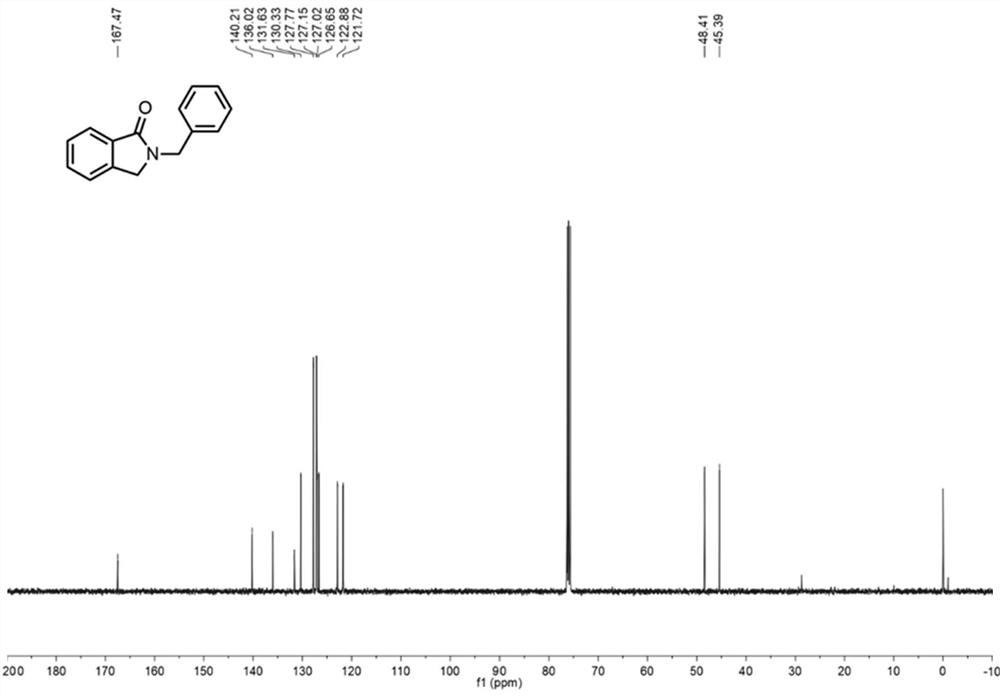
[0045] Embodiment 1: the synthesis of compound 2a
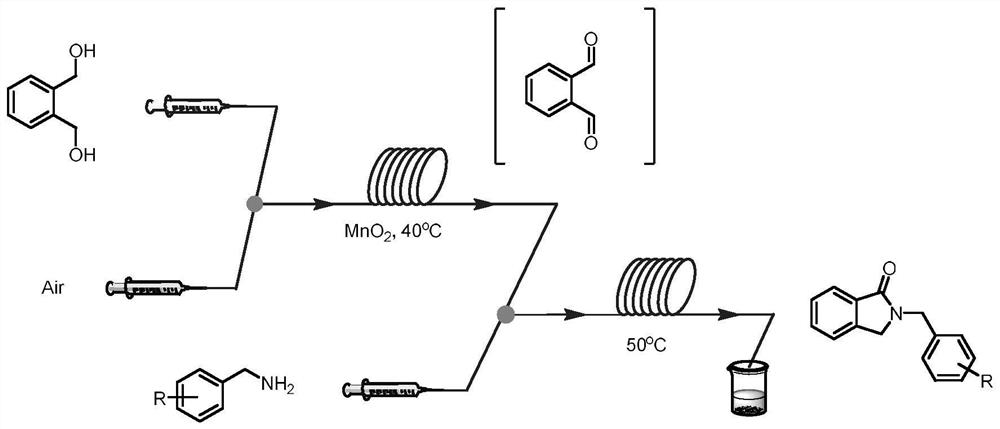
[0046] Inject 10 mL of aqueous solution of 1 mmol of ophthalmic alcohol, alcohol oxidase (700 U), flavin adenine dinucleotide (FAD, 6 μmol, 5 mg), and hydrogen peroxide reductase (0.02 μmol, 4 mg) into syringe A , air is drawn into syringe B, and the flow rate of the two samples is 56.25 μL min -1 After being pumped into the mixer, it is filled with nano MnO2 The column microreactor 1 was reacted at 40°C for 50min. Inject the acetonitrile solution of benzylamine 1a (1 mmol, 0.1 M) into syringe C and the effluent from microreactor 1 through the mixer at a flow rate of 56.25 μL min -1 Pump into coil microreactor 2 and react at 50°C for 5min. Add appropriate amount of water and ethyl acetate to the collected reaction solution for extraction, dry over anhydrous sodium sulfate, and concentrate under reduced pressure to obtain the crude product, which can be separated by column chromatography to obtain the target product 2a with ...
Embodiment 2
[0047] Embodiment 2: the synthesis of compound 2b
[0048] Inject 10 mL of aqueous solution of 1 mmol of ophthalmic alcohol, alcohol oxidase (700 U), flavin adenine dinucleotide (FAD, 6 μmol, 5 mg), and hydrogen peroxide reductase (0.02 μmol, 4 mg) into syringe A , air is drawn into syringe B, and the flow rate of the two samples is 56.25 μL min -1 After being pumped into the mixer, it is filled with nano MnO 2 The column microreactor 1 was reacted at 40°C for 50min. Inject the acetonitrile solution of 2-methylbenzylamine 1b (1mmol, 0.1M) into syringe C and the effluent from microreactor 1 through the mixer at a flow rate of 56.25 μL min -1 Pump into coil microreactor 2 and react at 50°C for 5min. An appropriate amount of water and ethyl acetate were added to the collected reaction solution for extraction, dried over anhydrous sodium sulfate, concentrated under reduced pressure to obtain a crude product, and separated by column chromatography to obtain the target product 2b...
Embodiment 3
[0049] Embodiment 3: the synthesis of compound 2c
[0050] Inject 10 mL of aqueous solution of 1 mmol of ophthalmic alcohol, alcohol oxidase (700 U), flavin adenine dinucleotide (FAD, 6 μmol, 5 mg), and hydrogen peroxide reductase (0.02 μmol, 4 mg) into syringe A , Air was sucked into syringe B, and the two samples were pumped into the mixer at a flow rate of 56.25 μL min-1 and then entered into the column microreactor 1 filled with nanometer MnO2 to react at 40°C for 50 minutes. Inject the acetonitrile solution of 4-methoxybenzylamine 1c (1mmol, 0.1M) into the syringe C and pump the effluent from the microreactor 1 into the coil microreactor 2 through the mixer at a flow rate of 56.25 μL min-1. React at 50°C for 5 minutes. Add appropriate amount of water and ethyl acetate to the collected reaction solution for extraction, dry over anhydrous sodium sulfate, and concentrate under reduced pressure to obtain a crude product, which can be separated by column chromatography to obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com