Method for preparing metal compound through anode metal electrooxidation and coupling hydrogen production
A metal compound, electro-oxidation technology, applied in the field of electrochemical materials, can solve problems such as waste water discharge, and achieve the effects of reducing costs, promoting oxidation, and reducing separation load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
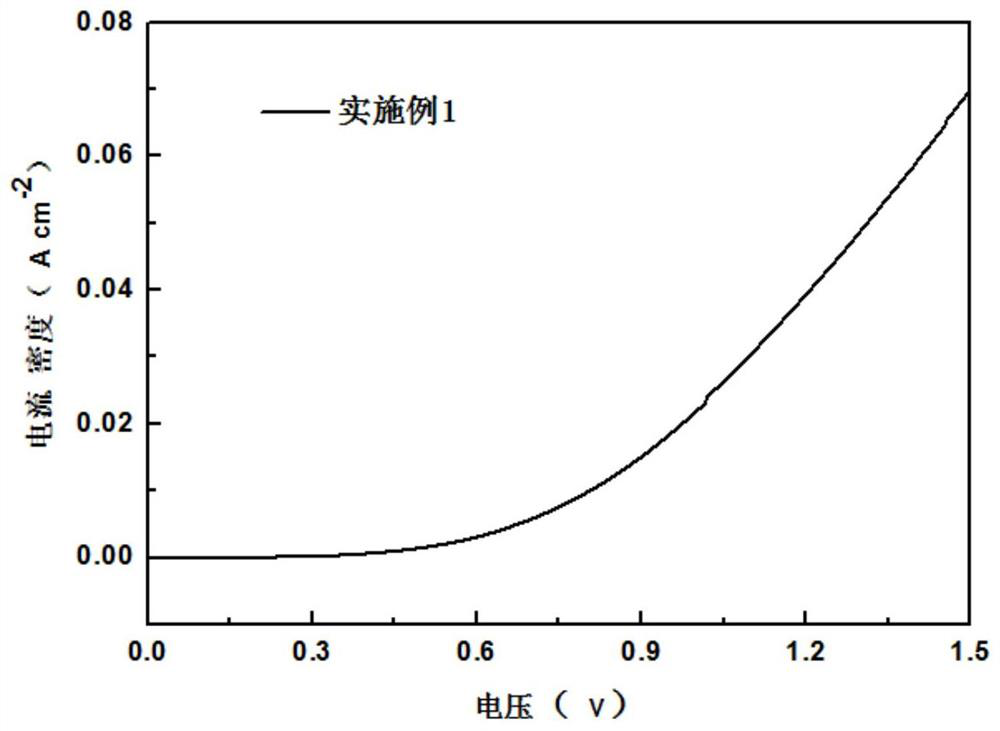
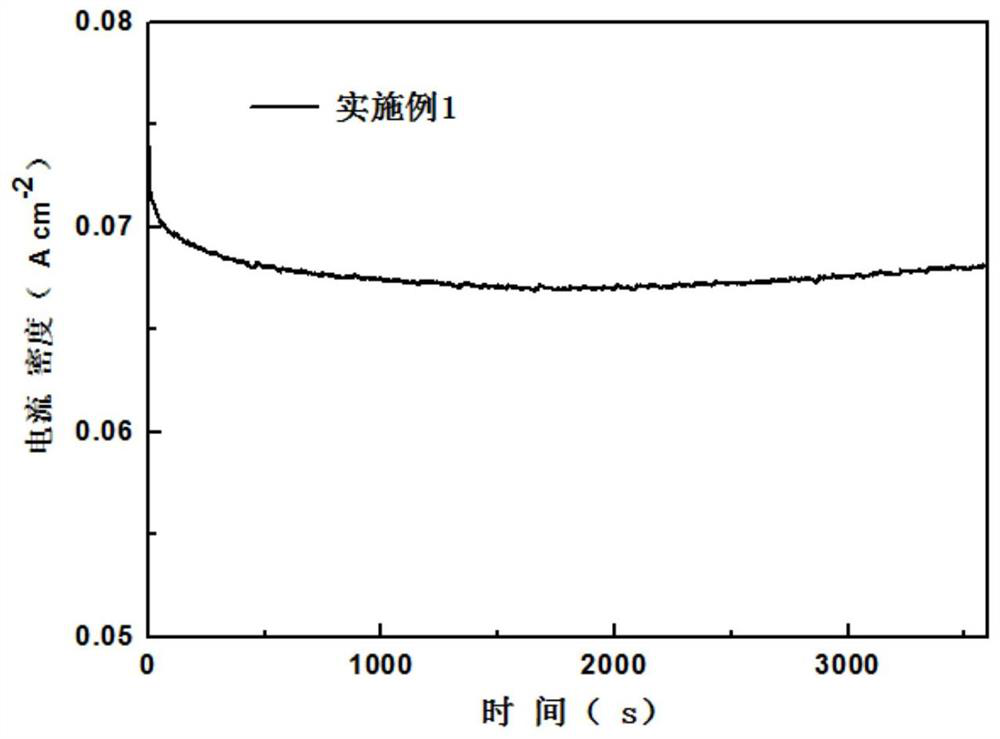
[0032] (1) Using the iron sheet as the working electrode, cut the high-purity iron sheet with a thickness of 0.5mm into 1×1.5cm 2 strips, with about 1×0.5cm reserved at the upper end 2 Connect with the platinum electrode holder, the lower end is 1×1cm 2 contact with electrolyte. The counter electrode and reference electrode use 1×2cm 2 Platinum sheet electrode, the electrolyte is 0.5M sodium chloride solution, and no electrocatalyst is loaded on the electrode. The electrolysis adopts a two-electrode system, so the potential set by the electrochemical workstation is the tank pressure, and the electrolysis process is accompanied by 400r / min magnetic stirring. First, a cyclic voltammetry scan was performed in the 0-1.5V potential interval (10 cycles, 50mV s -1 ) to activate the iron sheet until the curve is relatively stable. followed by 10mV s -1 A linear voltammetric scan was performed at a scan rate of 0-1.5V, and the polarization curve 1 was obtained. It can be seen tha...
Embodiment 2
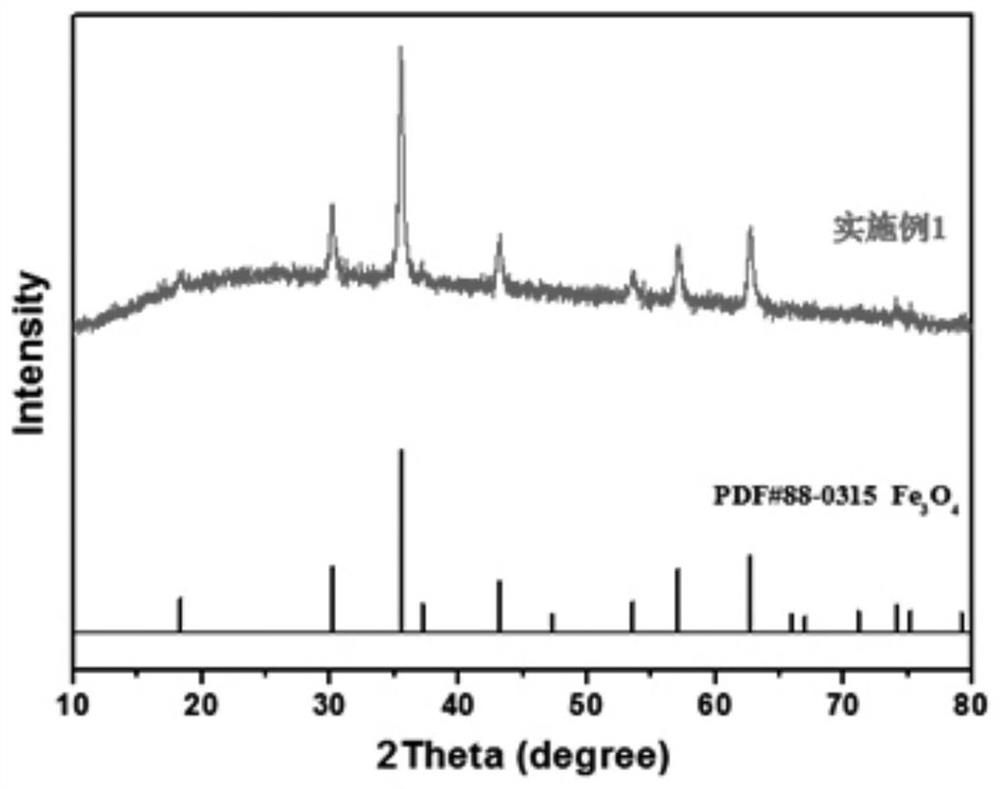
[0038] (1) Using the iron sheet as the working electrode, cut the high-purity iron sheet with a thickness of 0.5mm into 1×1.5cm2 strips, with about 1×0.5cm reserved at the upper end 2 Connect with the platinum electrode holder, the lower end is 1×1cm 2 contact with electrolyte. The counter electrode and reference electrode use 1×2cm 2 Platinum sheet electrode, the electrolyte is 0.5M sodium chloride solution, and the surfactant CTAB is added to the electrolyte, and its concentration reaches above CMC. No electrocatalyst was loaded on the electrodes, and the electrolysis process was accompanied by 400r / min magnetic stirring. First, a cyclic voltammetry scan was performed in the 0-1.5V potential interval (10 cycles, 50mV s -1 ) to activate the iron sheet until the curve is relatively stable. Electrolysis was then performed at a constant potential of 1.5 V for 1 hour. After the electrolysis was completed, a large amount of brown precipitate was produced in the solution, whic...
Embodiment 3
[0044] (1) Using the iron sheet as the working electrode, cut the high-purity iron sheet with a thickness of 0.5mm into 1×1.5cm 2 strips, with about 1×0.5cm reserved at the upper end 2 Connect with the platinum electrode holder, the lower end is 1×1cm 2 contact with electrolyte. The counter electrode and reference electrode use 1×2cm 2 Platinum electrode, the electrolyte is 0.5M sodium chloride + 0.01M lithium dihydrogen phosphate solution. No electrocatalyst was loaded on the electrodes, and the electrolysis process was accompanied by 400r / min magnetic stirring. First, a cyclic voltammetry scan was performed in the 0-1.0V potential interval (10 cycles, 50mV s -1 ) to activate the iron sheet until the curve is relatively stable. Electrolysis was then performed at a constant potential of 1.0 V for 1 hour. After the electrolysis is completed, a large amount of off-white precipitates are produced in the solution, which are not ferromagnetic. The precipitate was centrifuged...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com