2, 6-naphthalic acid-based copolyester material and preparation method thereof
A technology of naphthalene dicarboxylic acid-based copolyester and copolyester, which is applied in the field of 2,6-naphthalene dicarboxylic acid-based copolyester material and its preparation, can solve problems such as restrictions and environmental pollution, achieve difficult movement and expand application range, effect of reducing crystallinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
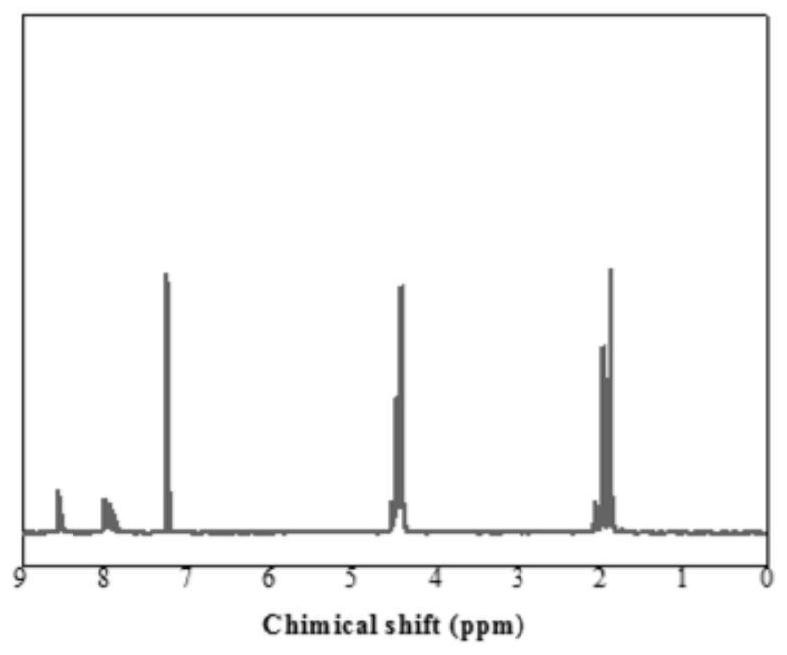
[0035] (1) Under the action of stannous octoate, 2,6-dimethyl naphthalene dicarboxylate, 2,5-dimethyl furandicarboxylate, 1,4-butanediol are added to the reaction flask, 1,4 -Butanediol and total dimethyl esters (dimethyl 2,6-naphthalene dicarboxylate and dimethyl 2,5-furandicarboxylate) in a molar ratio of 2:1, dimethyl 2,5-furandicarboxylate and The molar ratio of dimethyl 2,6-naphthalene dicarboxylate is 0.75:0.25. The stannous oxalate accounted for 0.5% of the mass percentage of the diester, under the protection of nitrogen, stirred and reacted at 260° C. for 7 hours to form a prepolymer.
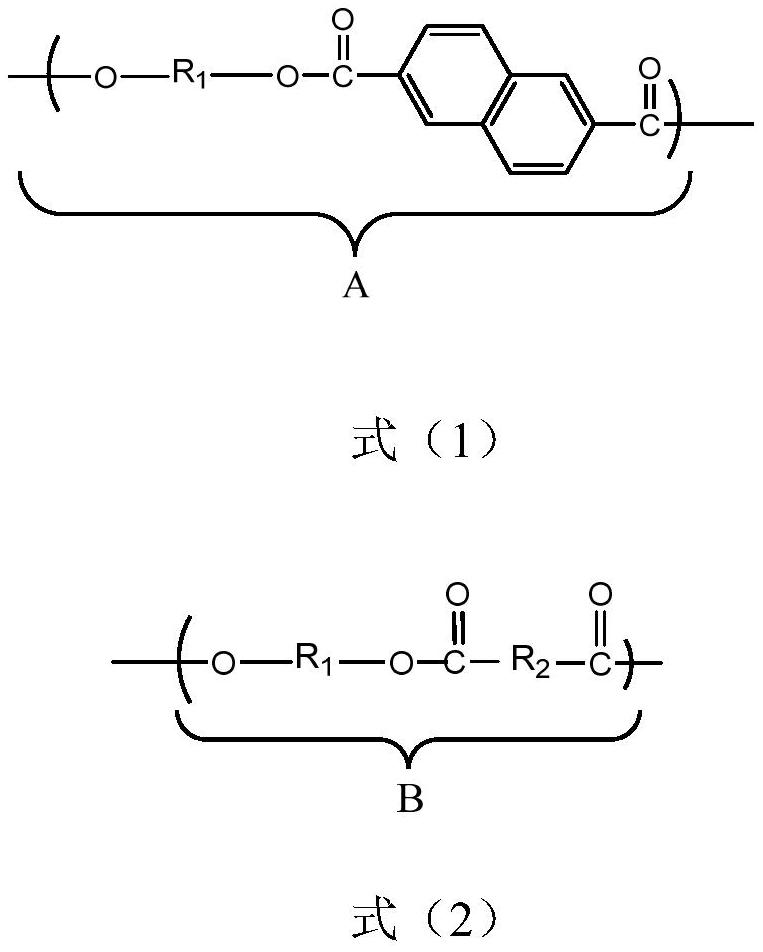
[0036] (2) Vacuumize the above-mentioned prepolymer to 80Pa, stir and react at 260°C for 3h to obtain copolyester. The structural formula is as follows:
[0037]
[0038]
[0039] R 1 for -CH 2 -CH 2 -CH 2 -CH 2 -;
[0040] R 2 for
[0041] Tensile properties (tensile strength and elongation at break) test conditions: test according to ASTM D-638 standard, dumbbell-sha...
Embodiment 2
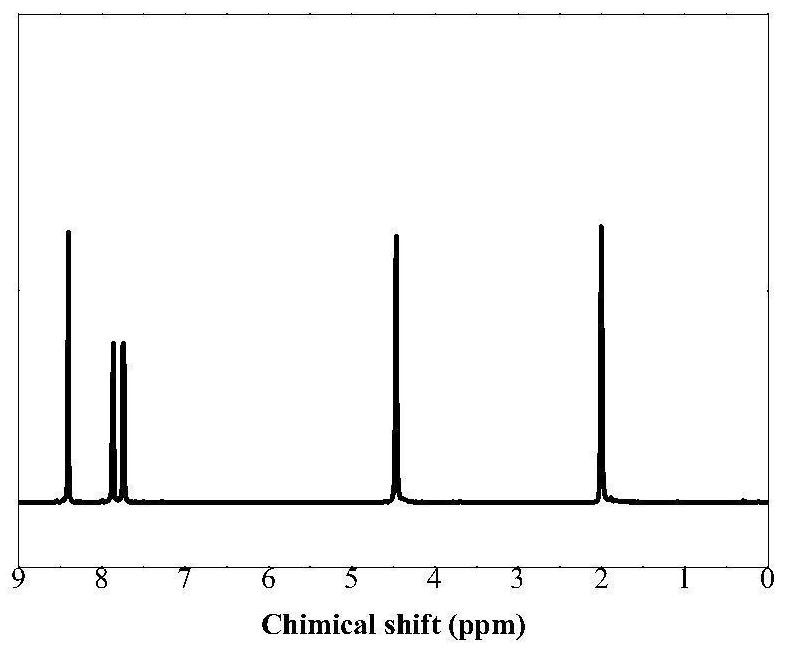
[0048] (1) under the action of tetrabutyl titanate, 2,6-naphthalene dicarboxylic acid, 2,5-thiophenedicarboxylic acid, 1,4-butanediol are added in the reaction flask, 1,4-butanediol and The molar ratio of total dicarboxylic acids (2,6-naphthalene dicarboxylic acid and 2,5-thiophenedicarboxylic acid) is 3:1, and the molar ratio of 2,5-thiophenedicarboxylic acid and 2,6-naphthalene dicarboxylic acid is 0.23:0.77 . The stannous oxalate accounted for 0.3% of the mass percentage of the diester, under the protection of nitrogen, stirred and reacted at 250° C. for 7 hours to form a prepolymer.
[0049] (2) Vacuumize the above prepolymer to 80Pa, stir and react at 250v for 3h to obtain copolyester. The structural formula is as follows:
[0050]
[0051] R 1 for -CH 2 -CH 2 -CH 2 -CH 2 -;
[0052] R 2 for
[0053] Tensile performance test conditions: the same as in Example 1, and the tensile properties are shown in Table 1. The oxygen and carbon dioxide permeability tes...
Embodiment 3
[0056] (1) under the effect of stannous octoate, 2,6-naphthalene dicarboxylic acid, 2,5-furandicarboxylic acid, 1,3-propanediol are added in the reaction flask, 1,3-propanediol and total diformic acid (2 , 6-naphthalene dicarboxylic acid and 2,5-furandicarboxylic acid) in a molar ratio of 3:1, 2,5-furandicarboxylic acid and 2,6-naphthalene dicarboxylic acid in a molar ratio of 0.74:0.26. The stannous oxalate accounted for 0.01% of the mass percentage of the diester, under the protection of nitrogen, stirred and reacted at 250° C. for 7 hours to form a prepolymer.
[0057] (2) Vacuumize the above-mentioned prepolymer to 80Pa, stir and react at 260°C for 3h to obtain copolyester. The structural formula is as follows:
[0058]
[0059]
[0060] R 1 for -CH 2 -CH 2 -CH 2 -;
[0061] R 2 for
[0062] Tensile performance test conditions: the same as in Example 1, and the tensile properties are shown in Table 1. The oxygen and carbon dioxide permeability test conditi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com