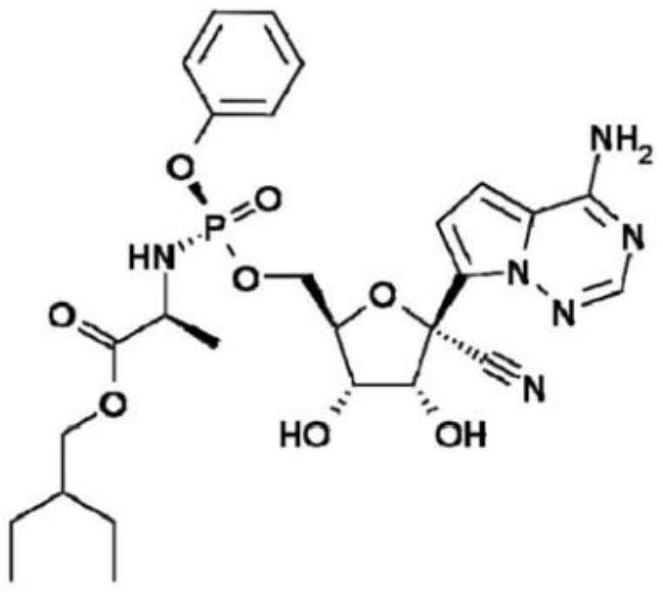
Redefovir composition and application thereof
A technology for Remdesivir and a composition, applied in the field of pharmaceutical preparations, can solve the problems of high packaging requirements, contaminated drugs, and high equipment requirements, and achieve the effects of simple preparation process, overcoming poor solubility, and satisfying drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Preparation of sulfobutyl-β-cyclodextrin solution
[0031] Weigh 2.5g of sulfobutyl-β-cyclodextrin into a 50mL volumetric flask, add 40mL of purified water, shake to dissolve, add an appropriate amount of hydrochloric acid solution to adjust the pH of the system to 1.8, and add purified water to constant volume to prepare 5 % (w / v) of sulfobutyl-β-cyclodextrin solution.
[0032] (2) Remdesivir Saturation Solubility Experiment
[0033] After remdesivir passes through an 80-mesh sieve, weigh 10 mg of remdesivir into a 5mL centrifuge tube, and add 0.8mL of the 5% (w / v) sulfobutyl-β-cyclodextrin solution prepared in step (1) , add 0.2mL of purified water, shake and disperse, add 2M hydrochloric acid to adjust the pH to 1.8, vortex mix for 50min, then add 3M sodium hydroxide solution to adjust the pH to about 3.5, then vortex mix for 50min, the suspension is centrifuged to obtain sulfobutyl Based on the Redcivir composition with a β-cyclodextrin content of 4% (w / v), th...
Embodiment 2
[0035] (1) Preparation of solubilizer solution
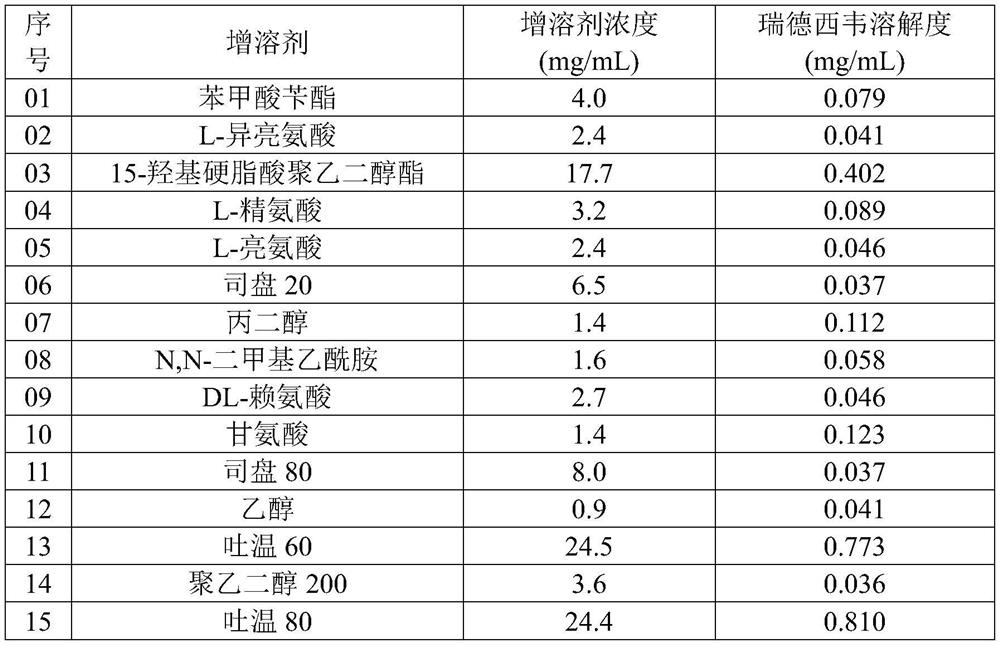
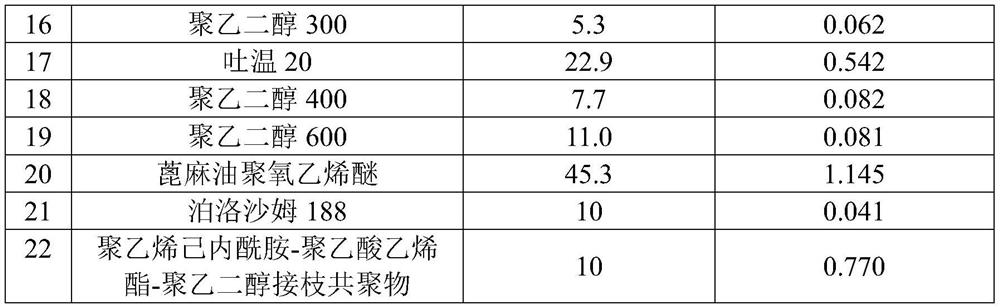
[0036] Weigh appropriate amounts of 22 kinds of solubilizers into 5mL test tubes, add 2mL of water, and prepare solubilizer solutions with appropriate concentrations (as shown in Table 1).
[0037] (2) Dissolution experiment of remdesivir
[0038] After remdesivir passes through an 80-mesh sieve, weigh 10 mg of remdesivir into a 5mL centrifuge tube, add 0.8mL of hydrochloric acid solution with a pH of 1.8 and 0.2mL of a solubilizer aqueous solution, shake and disperse, and then add 2M hydrochloric acid solution to adjust The pH is 1.8, vortexed for 50 minutes, then added 3M sodium hydroxide solution to adjust the pH to 3.5, vortexed for 50 minutes, and the suspension was centrifuged to obtain the remdesivir solubilizer composition, and the content of remdesivir in it was detected , to obtain the solubility of remdesivir in different solubilizer solutions, and the results are shown in Table 1.
[0039] Table 1
[0040]
[0...
Embodiment 3
[0044] Pass remdesivir through an 80-mesh sieve, weigh 10 mg into a 5 mL centrifuge tube, add 0.8 mL of the sulfobutyl-β-cyclodextrin solution prepared in the same way as in step (1) of Example 1, and adjust the pH to 1.8. Vortex for 50 min, add 0.2 mL of different types of solubilizer solutions prepared according to the same method as in Example 2 step (1), adjust the pH to 1.8, vortex for 50 min, adjust the pH of the system to about 3.5 with 3M sodium hydroxide solution, and vortex After 50 minutes, the suspension was centrifuged to obtain a remdesivir composition containing sulfobutyl-β-cyclodextrin and a solubilizer, and the content of remdesivir in it was determined, and the results are shown in Table 2.
[0045] Table 2
[0046]
[0047]
[0048] The results showed that after remdesivir and sulfobutyl-β-cyclodextrin were fully mixed, adding solubilizers L-isoleucine, L-arginine, Span 20, propylene glycol, Span 80, polyethylene glycol Alcohol 200, polyethylene glyc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com