Fluorine-containing polysaccharide high-molecular compound and preparation method thereof
A polymer compound and polysaccharide technology, which is applied in the field of fluorine-containing polysaccharide polymer compounds and its preparation, can solve the problems of poor material stability, difficult reagent deoxygenation and fluorination, and low physical strength, so as to improve stability and strength, improve Physical and chemical properties, effects of good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] At 70 ° C oil bath, 0.161 g of chitosan, 4 ml acetonitrile, 6 ml of 20% KOH aqueous solution, 0.225 grams of 2-fluoronacyridine-4-formic acid placed under nitrogen protection The mixture was stirred in the tube for 3 hours, and the reaction formulation is as follows:
[0048]
[0049] After completion of the reaction, cooled to room temperature, 1 mole per liter of hydrochloride system was added to the aqueous phase pH test strip. Ethyl acetate and water were added, and the resulting organic phase was added to give a saturated brine, then anhydrous magnesium sulfate was dried, carnamed dry solvent, and 0.071 g of the product was divided into a silica gel chromatography column and 44% yield.
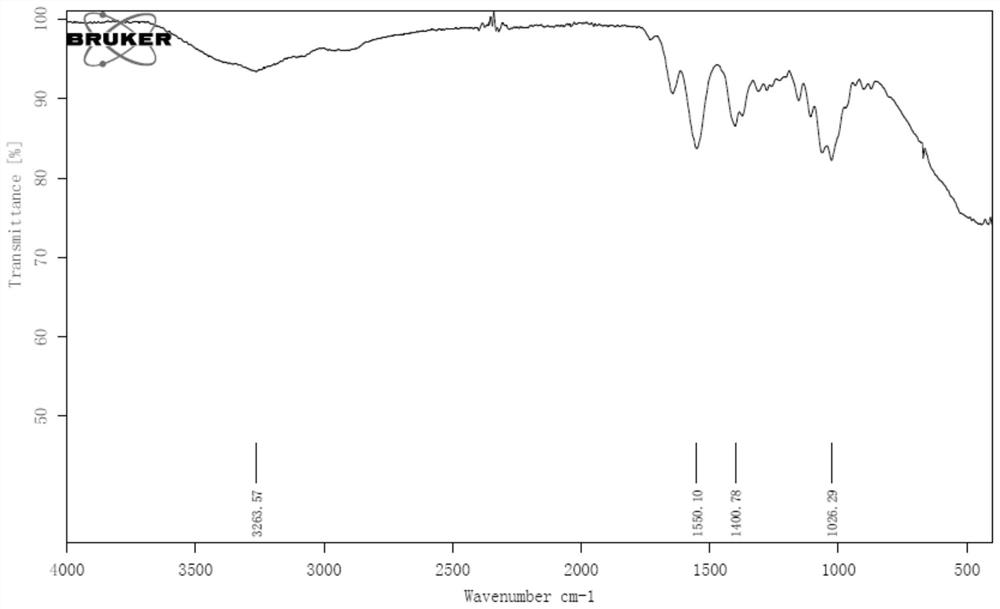
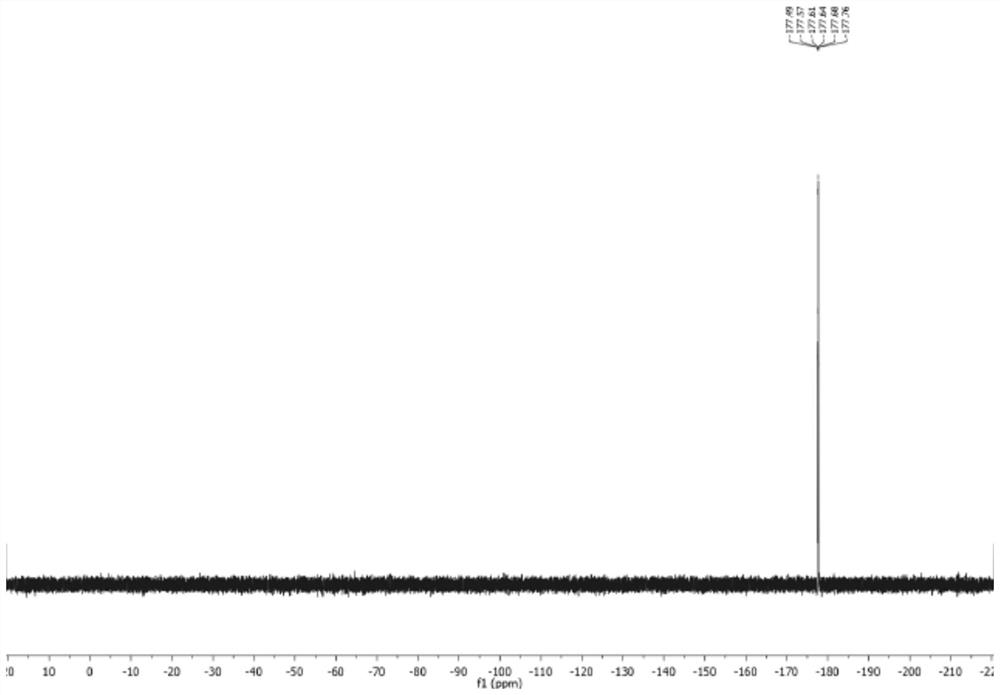
[0050] Infrared and flu fluoroscopy were performed on the preparation product, the result was IR (Thinfilm): 3263.57, 1550.10, 1400.78, 1300.55, 1026.29CM -1 , 19 F NMR (470MHz, H 2 O, 90 ° C, δ): - 177.7 (DT, J = 55.3, 34.3 Hz), the structural formula of the preparation product is a...
Embodiment 2
[0053] At 70 ° C oil bath, 0.161 g of glycan, 4 ml acetonitrile, 6 ml of 20% KOH aqueous solution, 0.225 grams of 5-fluoronacyridine-3-formic acid under nitrogen protection The mixture was stirred in the tube for 3 hours, and the reaction formulation is as follows:
[0054]
[0055] After completion of the reaction, cooled to room temperature, 1 mole per liter of hydrochloride system was added to the aqueous phase pH test strip. Ethyl acetate and water were added, and the obtained organic phase was added to give a saturated brine, then anhydrous magnesium sulfate was dried, carnamed dry solvent, divided by silica gel chromatography column, 0.049 g, 30% yield.
[0056] Infrared and flu fluoroscopy were performed on the preparation product, the result was IR (Thinfilm): 3263.57, 1550.10, 1400.78, 1300.55, 1026.29CM -1 , 19 F NMR (470MHz, H 2 O, 90 ° C, δ): - 177.7 (DT, J = 55.3, 34.3 Hz), the structural formula of the preparation product is as follows:
[0057]
Embodiment 3
[0059] At 70 ° C oil bath temperature, 0.161 g of chitosan, 4 ml acetonitrile, 6 ml of 20% KOH aqueous solution, 0.225 grams of 2-fluoronacyridine-6-formic acid were placed under nitrogen protection The mixture was stirred in the tube for 3 hours, and the reaction formulation is as follows:
[0060]
[0061] After completion of the reaction, cooled to room temperature, 1 mole per liter of hydrochloride system was added to the aqueous phase pH test strip. Ethyl acetate and water were added, and the obtained organic phase was added to give a saturated brine, then anhydrous magnesium sulfate was dried, carnamed dry solvent, divided by silica gel chromatography column, 0.022 grams, 13% yield.
[0062] Infrared and flu fluoroscopy were performed on the preparation product, the result was IR (Thinfilm): 3263.57, 1550.10, 1400.78, 1300.55, 1026.29CM -1 , 19 F NMR (470MHz, H 2 O, 90 ° C, δ): - 177.7 (DT, J = 55.3, 34.3 Hz), the structural formula of the preparation product is as follows...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com