GCA-NAb monoclonal antibody, hybridoma cell strain and application
A hybridoma cell line and monoclonal antibody technology, applied in the fields of medicine and biochemistry, can solve problems such as mandibular osteonecrosis, atypical fractures, and poor patient compliance, and achieve the effects of increasing mRNA levels, improving bone mass, and delaying development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1 immunogen preparation and animal immunization
[0043] (1) Amplify the sequence of the target gene as shown in SEQ ID No.2 by PCR, and insert the target gene into the corresponding expression vector; obtain a plasmid containing the correct sequence by sequencing; amplify the production of the plasmid and deliver downstream expression.
[0044] (2) Extract bacmid DNA from E coli cells containing recombinant bacmid, and obtain P1 recombinant baculovirus after infecting insect cells. The P1 generation virus was then transferred into sf9 insect cells to continue to amplify to obtain a high-titer P2 generation recombinant virus.
[0045] (3) Infect the high-five insect cells in the logarithmic growth phase with the high-titer P2 generation recombinant virus at a certain multiplicity of infection (MOI). Infected cells were further cultured at 27°C for 2-3 days to express the protein of interest.
[0046] (4) Use equilibration buffer to equilibrate the chromatog...
Embodiment 2
[0056] Example 2 blood collection and titer detection
[0057] One week after the last immunization, 50-60 μL of blood was collected through the orbital venous plexus of the mouse, and after standing at 4°C overnight, the upper serum was separated by centrifugation for inspection;
[0058] Dilute an appropriate amount of protein for detection to 5 μg / mL with coating buffer, then add 100 μL to each well of a 96-well plate with a single-channel pipette, tap the plate to mix the sample, seal it tightly with plastic wrap, and store at 4°C Coat overnight; wash the plate once with 200 μL / well of washing solution, and dry the plate; then seal the plate with 300 μL / well of blocking solution, and block for 1 hour at room temperature; wash the plate with 400 μL / well of washing solution Add the sample after 2 times (add 100 μL / well of the gradiently diluted sample and sample diluent), and add the detection antibody at the same time, add 100 μL / well into the 96-well plate, and let it reac...
Embodiment 3
[0059] Embodiment 3 fusion and screening
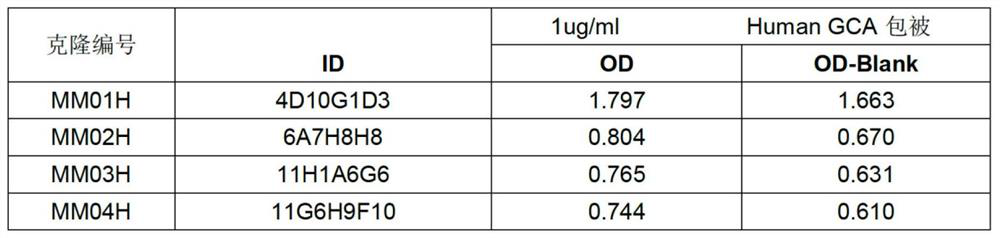
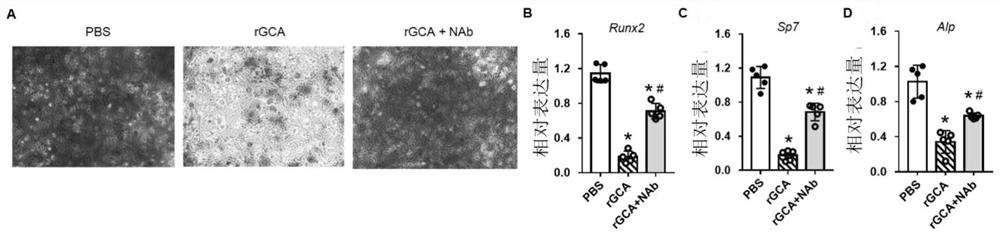
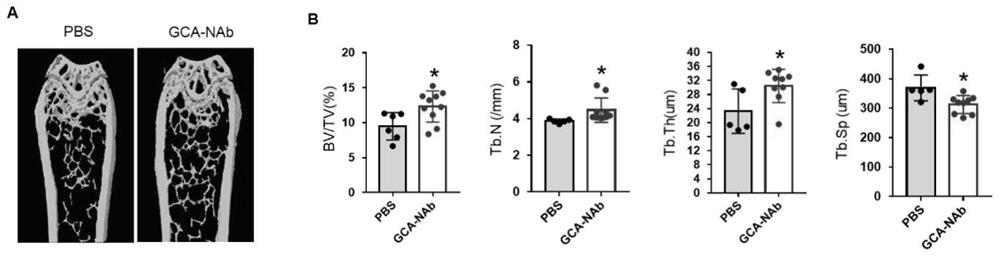
[0060] All the splenocytes of the immunized mice were collected, mixed with mouse myeloma cells at a ratio of 1:1, and fused by electrofusion to obtain hybridoma cells. Antigen protein was used for coating, cell supernatant was measured by ELISA method, and positive wells were selected for cloning by limiting dilution until a hybridoma cell line stably secreting monoclonal antibody was obtained. After screening, a total of 4 hybridoma cell lines were obtained. The supernatants were combined with the immunogen, that is, the monoclonal antibody GCA-Nab was successfully prepared (see figure 1 ), followed by selection of hybridoma cells with clone number MM04H for antibody preparation (preservation number is CCTCC NO: C2021182).
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com