Abamectin B2 derivatives as well as preparation method and application thereof
A technology of abamectin and derivatives, applied in the field of abamectin B2 derivatives and preparation thereof, can solve problems such as pollution, waste of resources, environment and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
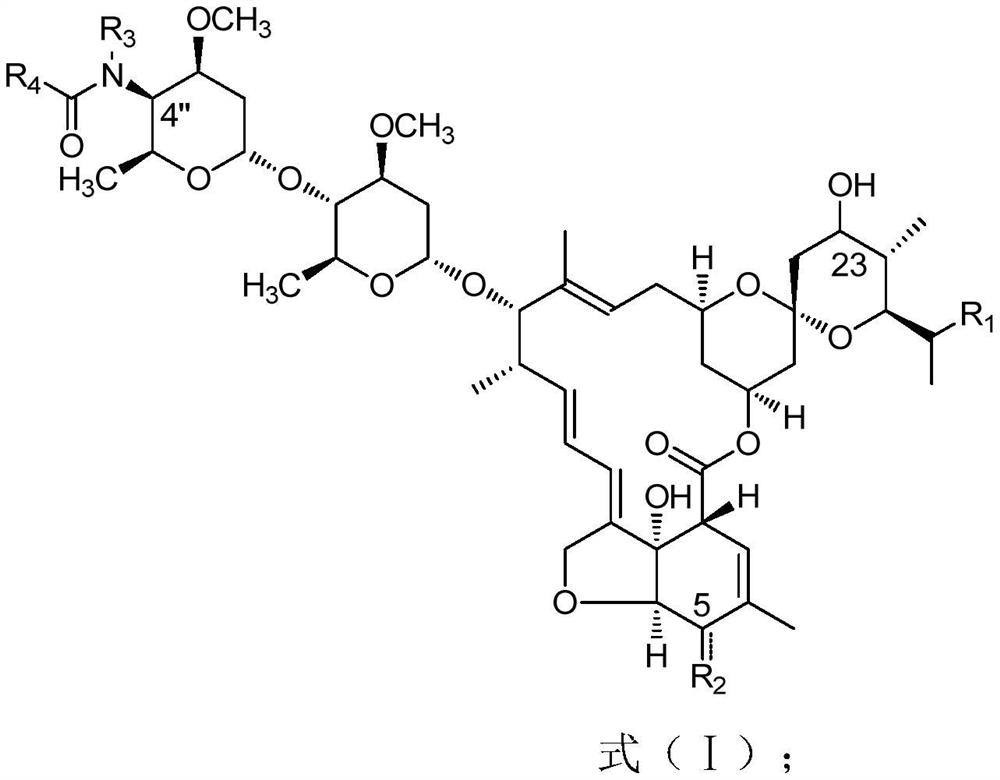
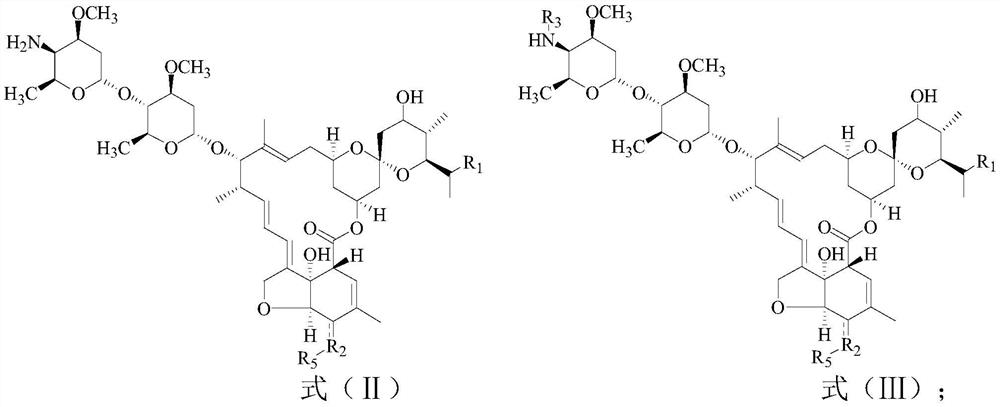
[0084] The present embodiment provides a kind of preparation method of the compound shown in formula (II):
[0085] When R2 is a hydroxyl group, it may comprise the following steps:
[0086] Take 10g of Abamectin B2a / 2b raw material and add it to 50g of dichloromethane, stir to dissolve, then add 1.7g of tetramethylethylenediamine, cool down to -15℃~-10℃, slowly add 1.6g of allyl chloroformate dropwise ester, after the dropwise addition, keep warm for 30min, then add 2.6g dimethyl sulfoxide, then slowly add 2.0g phenyl phosphate dichloride dropwise, keep warm for 1h, add 50mL water, stir at room temperature for 30min, then separate the phases, and separate the organic phase After drying over anhydrous magnesium sulfate, it was concentrated to dryness to obtain 10.3 g of light yellow solid;
[0087] Add the solid obtained in the previous step into 50mL of isopropyl acetate, stir to dissolve, add 0.5g of zinc acetate and 3.0g of hexamethyldisilazane, heat up and reflux for 6 ho...
Embodiment 2
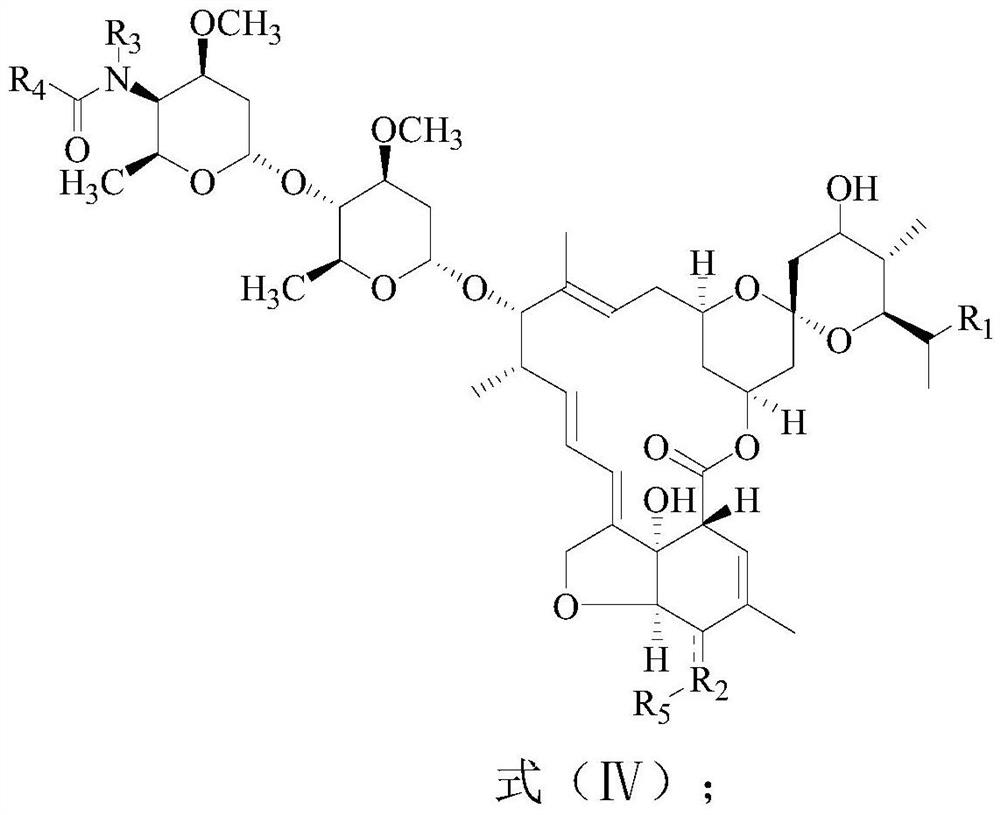
[0095] A preparation method of Abamectin B2 derivatives, comprising the following steps:
[0096] Step 1, the preparation of 4"-acetylamino-5-allyloxycarbonyl abamectin B2a / B2b:
[0097] 39.2g (40.8mmol) 4"-amino-5-allyloxycarbonyl Abamectin B2a / B2b (4"--amino-5-allyloxycarbonyl Abamectin B2a content greater than 95%) was added to In 120g of dichloromethane, cool the reaction solution to 0°C, add 5.2g (44.7mmol) tetramethylethylenediamine, under the protection of nitrogen, slowly add 3.5g of acetyl chloride (44.7mmol), and react at 0°C after the dropwise addition 2h, get the reaction solution containing 4"-acetylamino-5-allyloxycarbonyl abamectin B2a / B2b;
[0098] Take a small amount of the above reaction solution and confirm its structure after concentration and drying:
[0099] MS[M+H + ] (m / z): 1003.55.
[0100] 1 H NMR (500MHz, CDCl 3 )δ6.77(s,1H),5.96(ddt,J=16.6,10.4,6.2Hz,1H),5.78–5.70(m,2H),5.42(p,J=7.0Hz,1H),5.32–5.19 (m,4H),5.20–5.12(m,2H),5.03–4.95(m,3H),4.92–4....
Embodiment 3
[0111] A preparation method of Abamectin B2 derivative (5-hydroxylamine subunit-4 "-acetamido Abamectin B2a / B2b), comprising the following steps:
[0112] Step 1, 49.3g (0.05mol) 5-allyloxyimino-4 "-aminoabamectin B2a / B2b (5-allyloxyimino-4"-aminoabamectin B2a content Not less than 95%) was added to 446g of dichloromethane, the reaction solution was cooled to -5°C, 5.8g (0.05mol) of tetramethylethylenediamine was added, and 12.6g of trifluoroacetic anhydride (0.06mol) was slowly added, After the dropwise addition, the reaction was incubated and stirred for 30 minutes, and the remaining amount of 5-allyloxyimino-4"-aminoabamectin B2a / B2b was detected by high-performance liquid chromatography. The remaining amount of B2a / B2b was less than 1%. The reaction solution of hydroxylamine subunit-4"-acetamidoabamectin B2a / B2b;
[0113] Step 2. Cool the reaction solution containing 5-allyloxyhydroxylamine subunit-4"-acetamidoabamectin B2a / B2b in step 1 to 0°C, add 13g of methanol (0.4mo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com