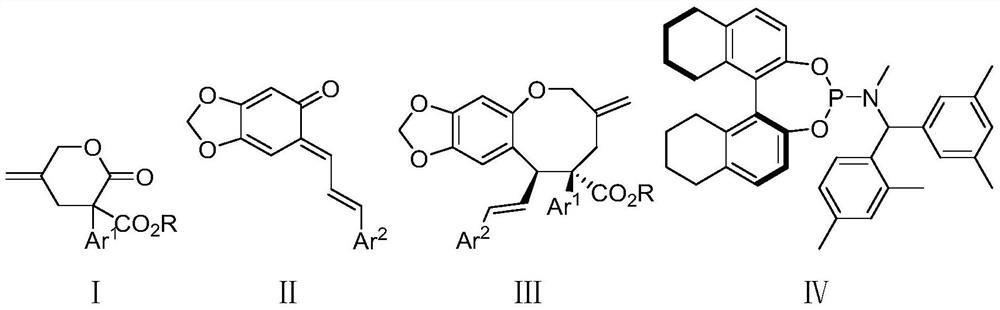
Method for synthesizing chiral oxygen-containing eight-membered ring compound through palladium-catalyzed asymmetric allyl cycloaddition reaction
An allyl ring, addition reaction technology, applied in asymmetric synthesis, organic chemical methods, chemical instruments and methods, etc., can solve the problems of no reports, etc., and achieve convenient and simple operation, high yield, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] (9S,10S)-9-(3,5-Dichlorophenyl)-7-methylene-10-((E)-styryl)-7,8,9,10-tetrahydro-6H- Synthesis of [1,3]bisoxazol[4',5':4,5]benzo[1,2-b]oxooctanoic acid-9-carboxylic acid methyl ester (Ⅲaa)
[0046] The reaction scheme is as follows:
[0047]
[0048] The operation steps are as follows:
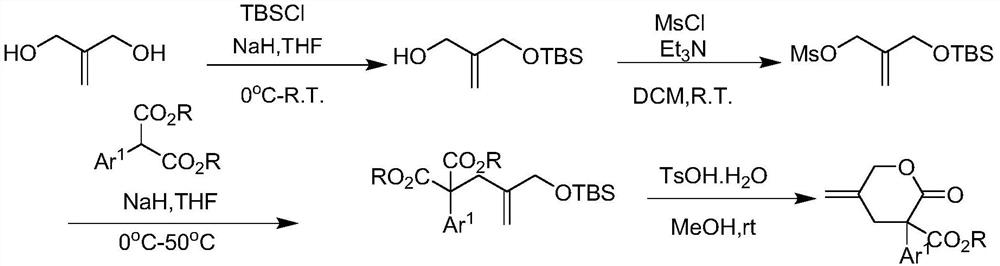
[0049] Under nitrogen protection, add Pd to a dry 5 mL reaction vial 2 (dba) 3 · CHCl 3 (0.005mmol, 5.2mg), chiral ligand (0.022mmol, 12.7mg) and 2mL of ethylbenzene, stirred at room temperature for 15 minutes, then transferred the resulting mixture to a 3-(3,5-dichlorophenyl )-5-methylene-2-oxytetrahydro-2H-pyran-3-carboxylic acid methyl ester Ia (63.0mg, 0.2mmol), (E)-6-((E)-3-phenylene Alkenyl)-benzo[d][1,3]dioxa-5-(6H)-one Ⅱa (50.4mg, 0.2mmol) and In a flask of molecular sieves (300mg), the reaction mixture was stirred and reacted at -20°C for 16h under nitrogen protection; the reaction solution was separated and purified by silica gel column chromatography (eluent: EA / PE=0...
Embodiment 2
[0056] (9S,10S)-9-(3,5-Dibromophenyl)-7-methylene-10-((E)-styryl)-7,8,9,10-tetrahydro-6H- Synthesis of [1,3]bisoxazol[4',5':4,5]benzo[1,2-b]oxoctanoic acid-9-carboxylate methyl ester (Ⅲba)
[0057] The reaction scheme is as follows:
[0058]
[0059] The operation steps are as follows:
[0060] Under nitrogen protection, add Pd to a dry 5 mL reaction vial 2 (dba) 3 · CHCl 3 (0.005mmol, 5.2mg), chiral ligand (0.022mmol, 12.7mg) and 2mL ethylbenzene, stirred at room temperature for 15 minutes, then transferred the resulting mixture to a 3-(3,5-dibromophenyl )-5-methylene-2-oxytetrahydro-2H-pyran-3-carboxylic acid methyl ester Ⅰb (80.8mg, 0.2mmol), (E)-6-((E)-3-phenylene Alkenyl)-benzo[d][1,3]dioxa-5-(6H)-one Ⅱa (50.4mg, 0.2mmol) and In a flask of molecular sieves (300mg), the reaction mixture was stirred and reacted at -20°C for 16h under nitrogen protection; the reaction solution was separated and purified by silica gel column chromatography (eluent: EA / PE=0.03-0.1:1,...
Embodiment 3
[0067] (9S,10S)-9-(4-Trifluoromethylphenyl)-7-methylene-10-((E)-styryl)-7,8,9,10-tetrahydro-6H- Synthesis of [1,3]bisoxazol[4',5':4,5]benzo[1,2-b]oxoctanoic acid-9-carboxylic acid methyl ester (Ⅲca)
[0068] The reaction scheme is as follows:
[0069]
[0070] The operation steps are as follows:
[0071] Under nitrogen protection, add Pd to a dry 5 mL reaction vial 2 (dba) 3 · CHCl 3 (0.005mmol, 5.2mg), chiral ligand (0.022mmol, 12.7mg) and 2mL ethylbenzene, stirred at room temperature for 15 minutes, then transferred the resulting mixture to a 3-(4-trifluoromethylphenyl )-5-methylene-2-oxytetrahydro-2H-pyran-3-carboxylic acid methyl ester Ic (62.8mg, 0.2mmol), (E)-6-((E)-3-phenylene Alkenyl)-benzo[d][1,3]dioxa-5-(6H)-one Ⅱa (50.4mg, 0.2mmol) and In a flask of molecular sieves (300mg), the reaction mixture was stirred and reacted at -20°C for 16h under nitrogen protection; the reaction solution was separated and purified by silica gel column chromatography (eluent: EA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com