Preparation method of 2-methyl-4-acetoxy-2-butenal with thermal stability
A technology of acetoxy and thermal stability, which is applied in the field of preparation of 2-methyl-4-acetoxy-2-butenal, can solve the problem of large loss, high content and affecting the effective yield of vitamin A synthesis reaction Physiological activity of vitamin A products and other issues to achieve the effect of improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
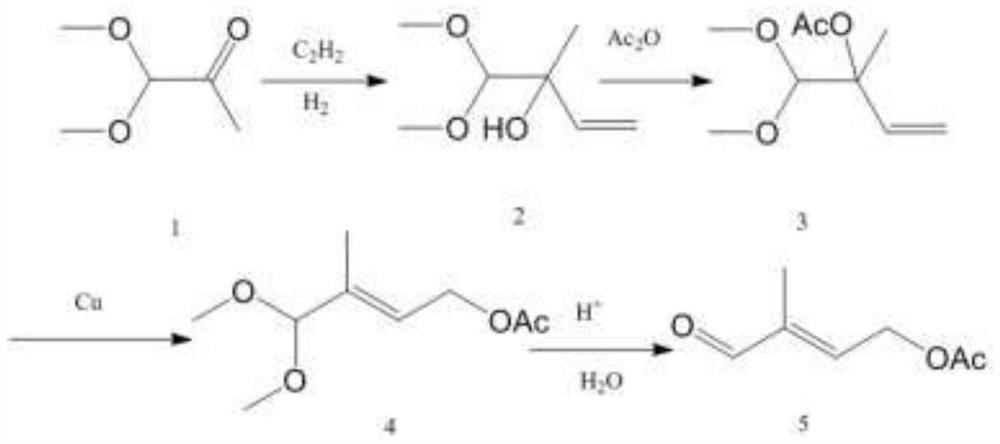
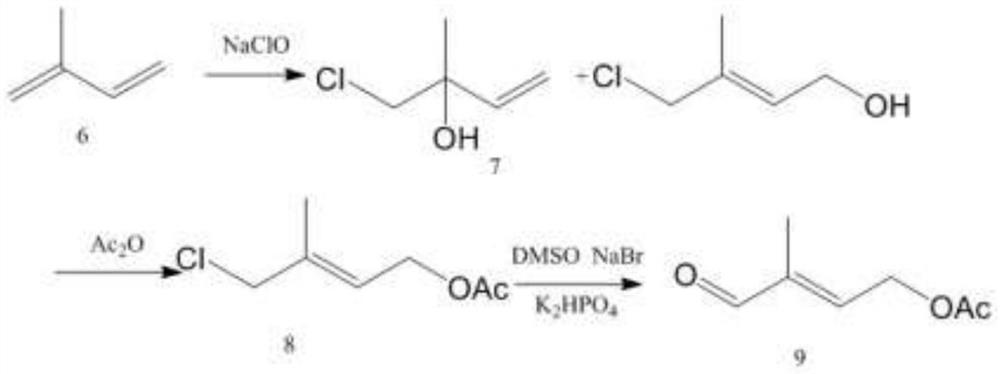
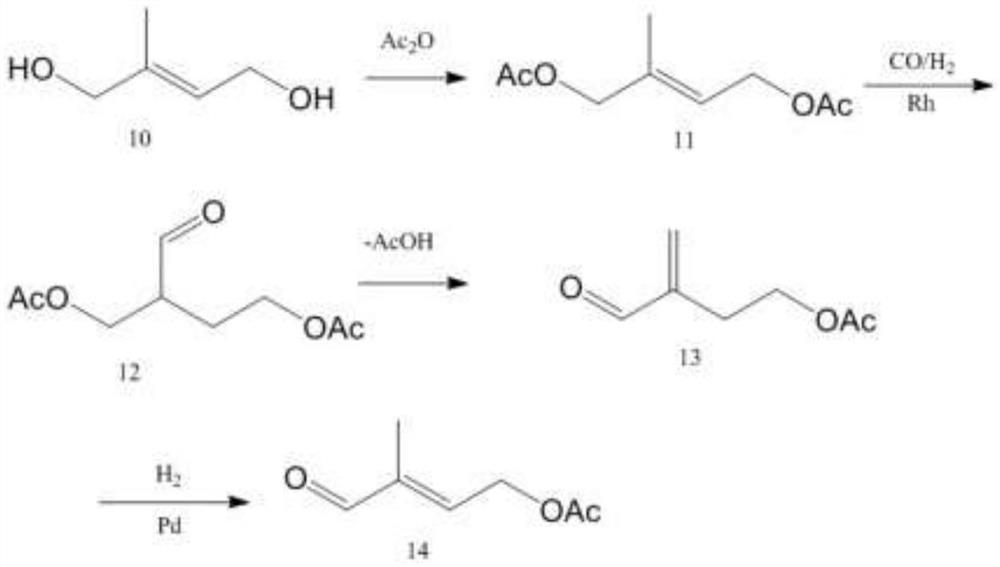
Image
Examples
Embodiment 1
[0059] Weigh 120kg xylene (active hydrogen content is 46mgKOH / kg in terms of hydroxyl value), 1000g 5%Pt / Al 2 o 3 Add the catalyst into a 500L bubbling kettle, raise the temperature to 90°C, add 40kg of the raw material 2-methylene-4-acetoxybutyraldehyde to the reaction kettle, and feed 200L / min Mixed gas (3%H 2 / 97%N 2 ) to start the reaction; after 6 hours of reaction, the temperature was lowered to 30° C. to stop the reaction, and the conversion rate of raw materials was measured to be 95.0%, the selectivity of trans-2-methyl-4-acetoxy-2-butenal was 83%, and the selectivity of cis The selectivity of 2-methyl-4-acetoxy-2-butenal is 0.8%; after the catalyst is filtered, the reaction solution is sequentially passed through the continuous solvent removal tower, by-product removal tower, raw material removal tower, and product tower separation system Solvent, hydrogenation by-products, unreacted raw material 2-methylene-4-acetoxybutyraldehyde, heavy components, product 2-meth...
Embodiment 2
[0063] Weigh 80kg toluene (the active hydrogen content is 15mgKOH / kg in terms of hydroxyl value), 890g 4.5%Pt / Al 2 o 3 Add the catalyst into a 500L bubbling kettle, raise the temperature to 50°C, add 40kg of the raw material 2-methylene-4-acetoxybutyraldehyde to the reaction kettle, and feed 89L / min Mixed gas (4%H 2 / 96%N 2 ) to start the reaction; after 7 hours of reaction, the temperature was lowered to 30° C. to stop the reaction, and the conversion rate of raw materials was measured to be 63%, the selectivity of trans 2-methyl-4-acetoxy-2-butenal was 92.7%, and the cis-2 - The selectivity of methyl-2-butenal is 0.8%; after the catalyst is filtered, the reaction solution passes through the solvent removal system, the by-product removal tower, the raw material removal tower, the product tower, and the hydrogenation by-product successively. The product, unreacted raw material 2-methylene-4-acetoxybutyraldehyde, heavy components, and the product 2-methyl-4-acetoxy-2-butenal...
Embodiment 3
[0067] Weigh 100kg toluene (the active hydrogen content is 27.3mgKOH / kg in terms of hydroxyl value), 800g 8%Pd / Al 2 o 3 Add the catalyst into a 500L bubbling kettle, raise the temperature to 90°C, add 40kg of the raw material 2-methylene-4-acetoxybutyraldehyde to the reaction kettle, and feed 200L / min Mixed gas (6%H 2 / 94%N 2 ) to start the reaction; after 4 hours of reaction, the temperature was lowered to 30° C. to stop the reaction, and the conversion rate of raw materials was measured to be 72%, the selectivity of trans-2-methyl-4-acetoxy-2-butenal was 93.1%, and the selectivity of cis The selectivity of 2-methyl-2-butenal is 0.8%. After the catalyst is filtered, the reaction solution passes through the solvent removal system, the solvent removal tower, the by-product removal tower, the raw material removal tower, and the product tower successively. By-products, unreacted raw materials 2-methylene-4-acetoxybutyraldehyde, heavy components, the yield of product 2-methyl-4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com