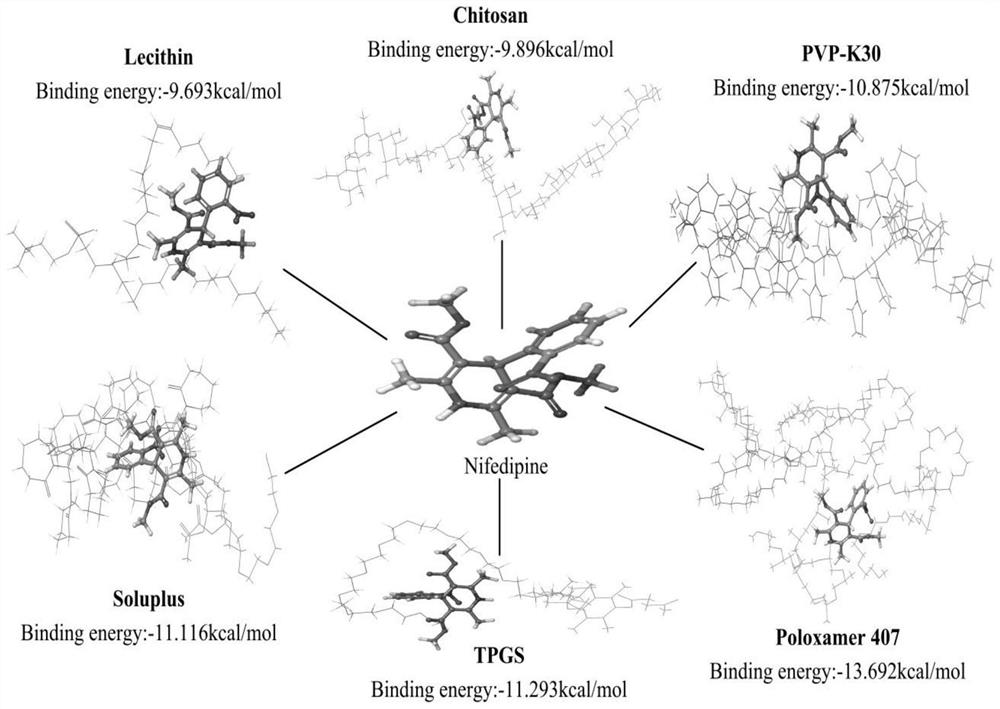
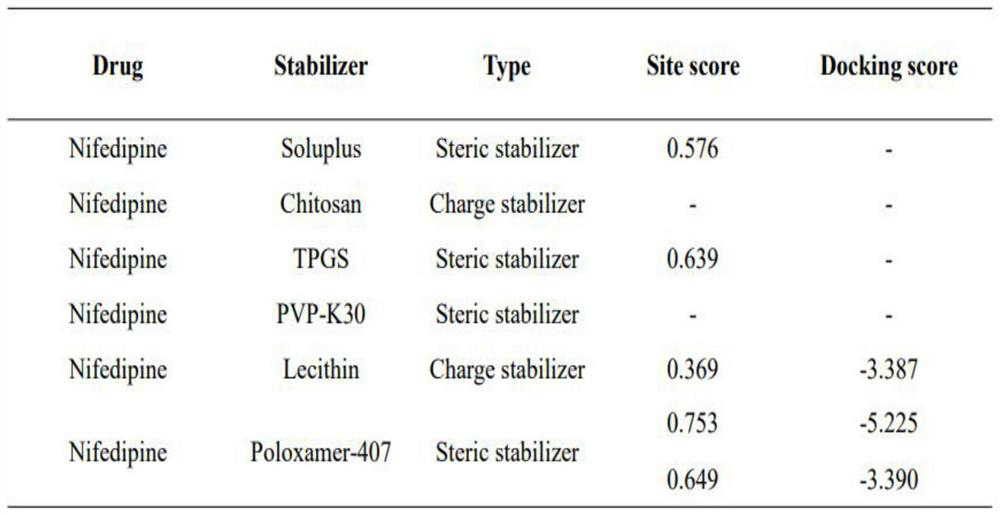
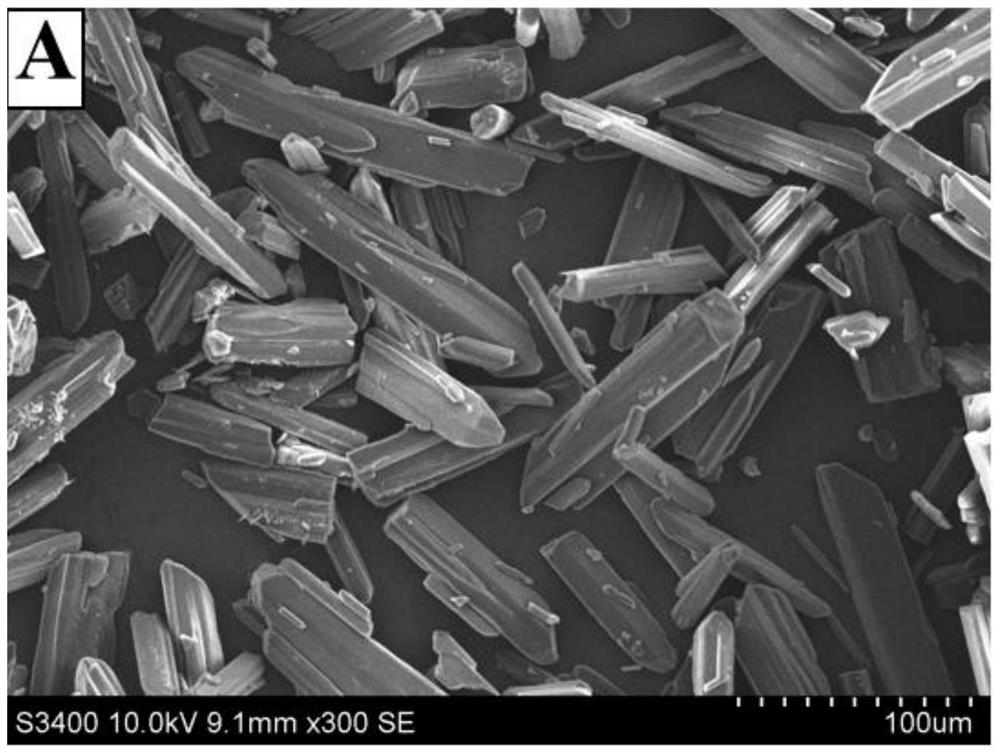
Dihydropyridine medicine nano suspension and preparation method thereof
A dihydropyridine, nanosuspension technology, applied in the directions of drug combination, nanotechnology, pharmaceutical formulations, etc., can solve the problems of improving the solubility of dihydropyridines without nanosuspension technology, and achieve good physical stability. , the effect of increasing the specific surface area and increasing the dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] The embodiment of the present invention also discloses a preparation method of the above-mentioned dihydropyridine drug nanosuspension, which specifically includes the following steps:
[0064] (1) Add the stabilizer to the water for 15-60 minutes to form a stabilizer solution;
[0065] (2) Add dihydropyridines to the above stabilizer solution, and magnetically stir for 30-90 minutes to obtain a coarse suspension;
[0066] (3) Add the coarse suspension into the grinding chamber of the ultrafine grinder, and add grinding beads at the same time, and grind to obtain the dihydropyridine drug nanosuspension;
[0067] Among them, the grinding beads are yttrium-stabilized zirconium beads, zirconium silicate grinding beads or polystyrene grinding beads, the particle size of the grinding beads is 0.3-0.8mm, and the volume of the grinding beads is 140ml; the grinding time is 15-60min, and the grinding speed It is 1500-4500rpm, and the grinding temperature is controlled at 10-30°...
Embodiment 1
[0071] Embodiment 1 of the present invention also discloses a preparation method of a dihydropyridine drug nanosuspension, specifically comprising the following steps:
[0072] (1) Take 0.1g of Soluplus, add it to 50ml of distilled water, stir with a magnetic stirrer for 30min, and the stirring speed is 600r / min to obtain a stabilizer solution;
[0073] (2) Add 0.25g nitrendipine to the stabilizer solution, stir with a magnetic stirrer for 60min, and the stirring speed is 450r / min to obtain a coarse suspension;
[0074] (3) Add the coarse suspension to the grinding chamber of the circulating nano-grinding machine, then add 140 ml of zirconia grinding beads with a particle size of 0.6-0.8 mm, and grind for 30 min at 3000 rpm to obtain the nitrendipine nano-suspension .
[0075] The prepared nitrendipine nanosuspension was diluted 3 times with purified water, and the measured particle size was 478.6nm, and the potential was -28.62mV.
Embodiment 2
[0077] Embodiment 2 of the present invention also discloses a preparation method of a dihydropyridine drug nanosuspension, specifically comprising the following steps:
[0078] (1) Take chitosan 0.1g, add in 50ml distilled water, stir with a magnetic stirrer for 30min, and the stirring speed is 600r / min to obtain a stabilizer solution;
[0079] (2) Add 0.25g nitrendipine to the stabilizer solution, stir with a magnetic stirrer for 60min, and the stirring speed is 450r / min to obtain a coarse suspension;
[0080] (3) Add the coarse suspension to the grinding chamber of the circulating nano-grinding machine, then add 140 ml of zirconia grinding beads with a particle size of 0.6-0.8 mm, and grind for 30 min at 3000 rpm to obtain the nitrendipine nano-suspension .
[0081] The prepared nitrendipine nanosuspension was diluted 3 times with purified water, and the measured particle size was 277.1 nm, and the potential was -6.55 mV.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com