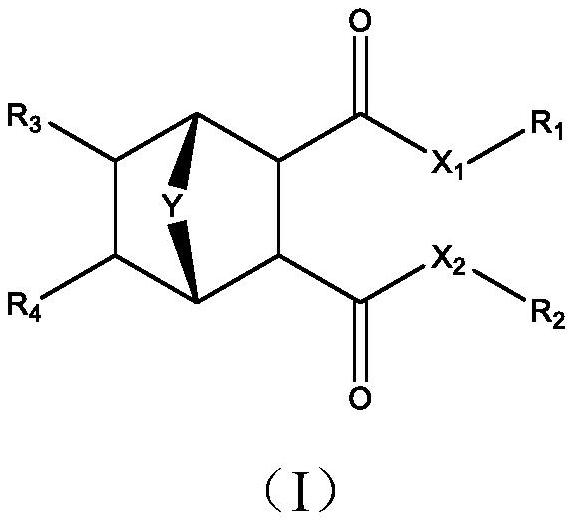
Cyclohexane dicarboxylic acid derivative with bridged ring, pharmaceutical composition comprising same, and application
A compound and cycloalkyl technology, applied in cyclohexanedicarboxylic acid derivatives and pharmaceutical compositions and application fields thereof, can solve the problems of high fat solubility, weak inhibitory effect, unfavorable oral preparations and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
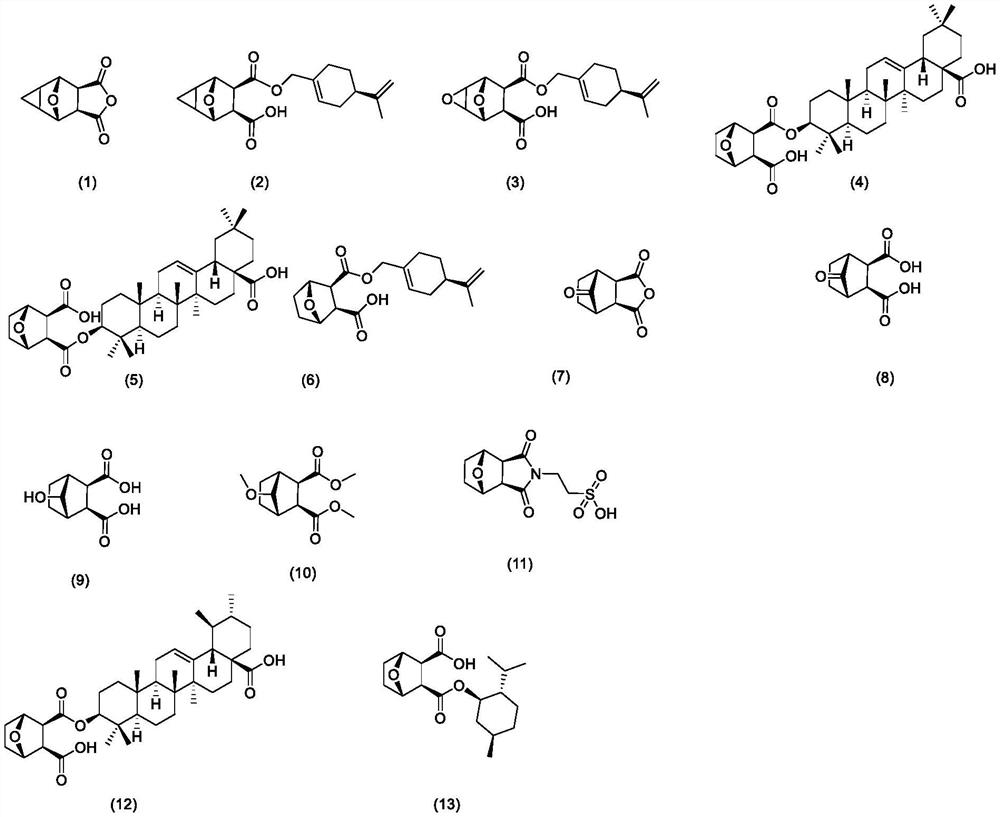
Image
Examples
Embodiment 1
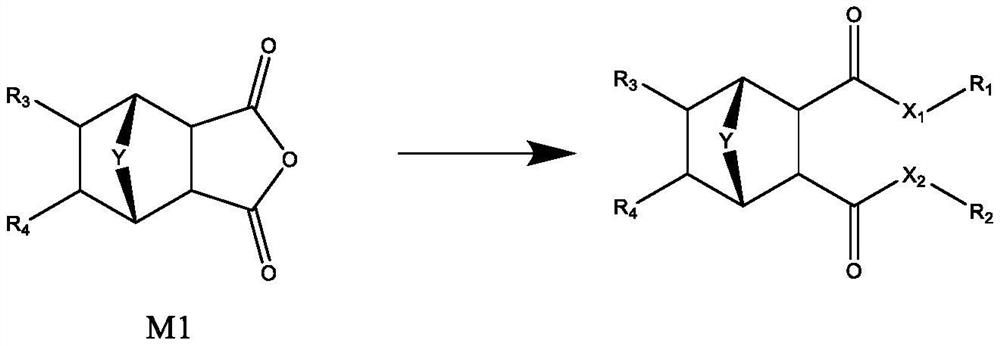
[0082] The cyclohexane dicarboxylic acid derivative of band bridged ring of the present invention can adopt following route preparation:
[0083]
[0084] (1) prepare nordehydrocantharidin
[0085] Weigh 20g (204mmol) of maleic anhydride, add it to a 250mL round bottom bottle, add 120mL of dry ether to dissolve, then slowly add 19g (279mmol) of furan dropwise, after the addition is complete, stir at room temperature for 24h. After suction filtration, the filter cake was evaporated to dryness to obtain 30 g (181 mmol) of white solid, with a yield of 89%. Mp:120-122℃;IR(KBr,cm -1 ):3050,1860,1795; 1 H NMR (CDCl 3 ,400MHz)δ:3.19(s,2H),5.44-5.47(m,2H),6.57-6.59(m,2H).
[0086] (2) preparation of nordehydrocantharidin dimethyl ester
[0087] Weigh 1.7g (10mmol) of nordehydrocantharidin, add it to a 25mL round-bottom bottle, then add 8mL of saturated hydrogen chloride methanol solution to reflux overnight, evaporate the solvent under normal pressure, add 10mL of distilled wa...
Embodiment 2
[0126] Antitumor Activity Test of Norcantharidin Derivatives in Vitro
[0127] The experiment used the MTT method to test the inhibitory activity of the test compound on different tumor lines (human liver cancer cell HepG2, gastric cancer cell BGC803, human lung cancer cell H460, blood cancer cell HL60, ovarian cancer cell HO8901). The test was divided into positive control group, blank control group and experimental group. . The positive group was added with corresponding concentrations of perillyl alcohol, oleanolic acid and norcantharidin as positive controls; the blank control group was added with DMSO (1%); the test group was added with corresponding concentrations of test compounds.
[0128] Take the above five tumor cells in the exponential growth phase, and adjust the concentration of the cell suspension to 1×10 with RPMI-1640 medium containing 10% calf serum. 4 cells / mL; inoculate in 96-well plate, add 100 μL cell suspension to each well, place in 5% CO 2 , Cultivat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com