Tablet containing azilsartan kamedoxomil and preparation method thereof
A technology for azilsartan medoxomil and potassium salt tablets, which is applied to medical preparations containing active ingredients, pill delivery, and pharmaceutical formulations, and can solve problems such as low bioavailability, low drug encapsulation rate, and fragile structure , to achieve the effect of simple preparation method, simple preparation method and improved shelf life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the preparation method that contains azilsartan medoxomil potassium salt tablet
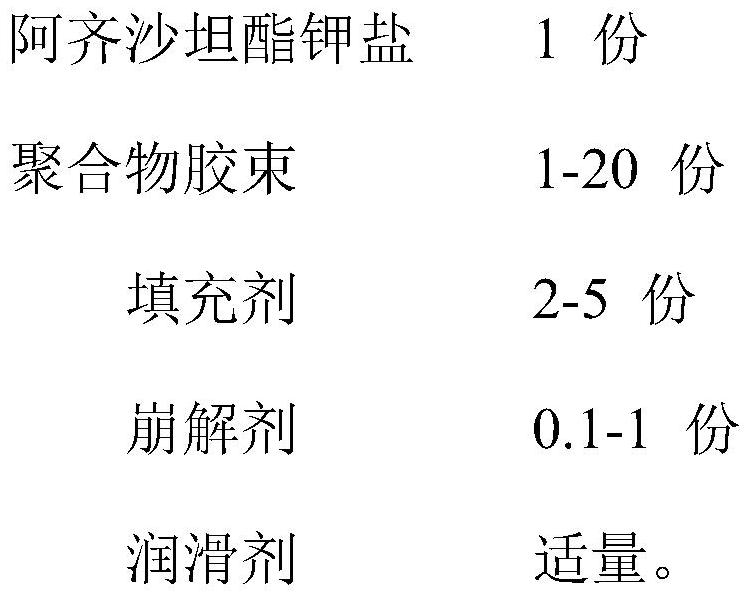
[0034] Composition Component parts by weight Azilsartan medoxomil potassium salt 10mg Polyethylene glycol 4000-polyglutamic acid 6000 60mg Crospovidone 40mg Mannitol 3mg Magnesium stearate 0.7mg
[0035] 1) Add the above-mentioned polyethylene glycol 4000-polyglutamic acid 6000 into the aqueous solution, mix well and set aside;
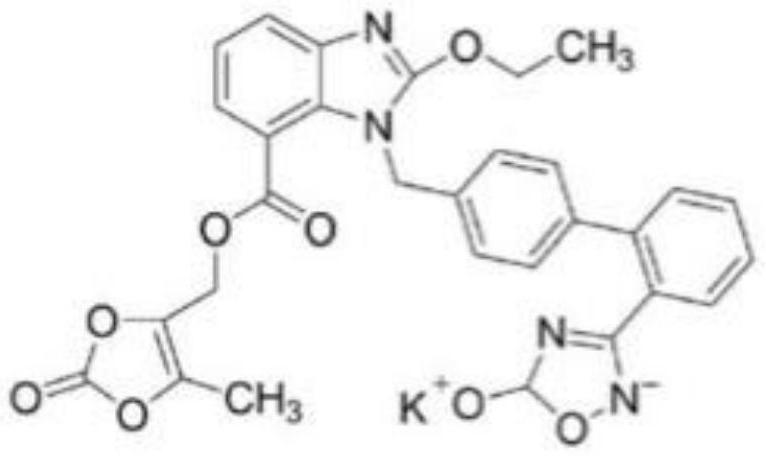
[0036] 2) Dissolving the above-mentioned azilsartan medoxomil potassium salt in methanol solution and stirring at room temperature to obtain azilsartan medoxomil potassium salt solution;
[0037] 3) Slowly add the azilsartan medoxomil potassium salt solution into the ethylene glycol 4000-polyglutamic acid 6000 aqueous solution, slowly spin evaporate to remove the methanol, and transfer the spin evaporated solution to a dialysis bag with a molecular weight cut off of 3000 for dialysis , dialyze in dis...
Embodiment 2
[0039] Embodiment 2: Azilsartan medoxomil potassium salt tablet
[0040] Composition Component parts by weight Azilsartan medoxomil potassium salt 10mg Polyethylene glycol 6000-polyglutamic acid 10000 40mg Crospovidone 30mg Mannitol 2mg Magnesium stearate 0.5mg
[0041] Concrete component and component weight are as above table, and concrete preparation method is the same as embodiment 1
Embodiment 3
[0042] Embodiment 3: Azilsartan medoxomil potassium salt tablet
[0043] Composition Component parts by weight Azilsartan medoxomil potassium salt 10mg Polyethylene glycol 6000-polyglutamic acid 12000 40mg Low-substituted hydroxypropyl cellulose 30mg Mannitol 2mg Magnesium stearate 0.4mg
[0044] Concrete component and component weight are as above table, and concrete preparation method is the same as embodiment 1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com