Tannase AfTan2.0 mutant and application thereof
A technology of tannase and mutants, applied in the field of molecular biology, can solve problems such as changes in fermentation temperature, influence of tannase catalytic activity, poor thermal stability of tannase, etc., and achieve the effect of improving thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The mutation strategy of embodiment 1 tannase AfTan2.0
[0020] The three-dimensional structure model of AfTan2.0 was constructed using the protein online modeling software SWISS-MODEL (https: / / swissmodel.expasy.org / interactive), and the crystal structure of Aspergillus niger tannase (PDB: 7k4o) was also selected as a template. The model constructed by SWISS-MODEL was optimized using the online tool GalaxyRefine (http: / / galaxy.seoklab.org / cgi-bin / submit.cgi?type=REFINE). Use PROCHECK to detect the quality of the model before and after optimization, and select the optimal model for molecular dynamics simulation. At a temperature of 400K, a 20ns molecular dynamics simulation of the protein was carried out using the software NAMD2.12 and 100 frames of structure were extracted from the simulated trajectory. FoldX4.0 software was used to perform scanning mutation operation on each frame of protein structure extracted from the molecular dynamics simulation trajectory, and th...
Embodiment 2
[0022] The mutant construction of embodiment 2 tannase AfTan2.0
[0023] The primers used to construct the mutants for the thermal stability study of AfTan2.0 are shown in Table 1, and the mutant primers were synthesized by Suzhou Jinweizhi Biotechnology Co., Ltd. The method of mutant construction adopts conventional methods.
[0024] Table 1 Mutant primers for thermostability studies
[0025]
[0026]
[0027] Note: The underlined mark is the mutation site
Embodiment 3
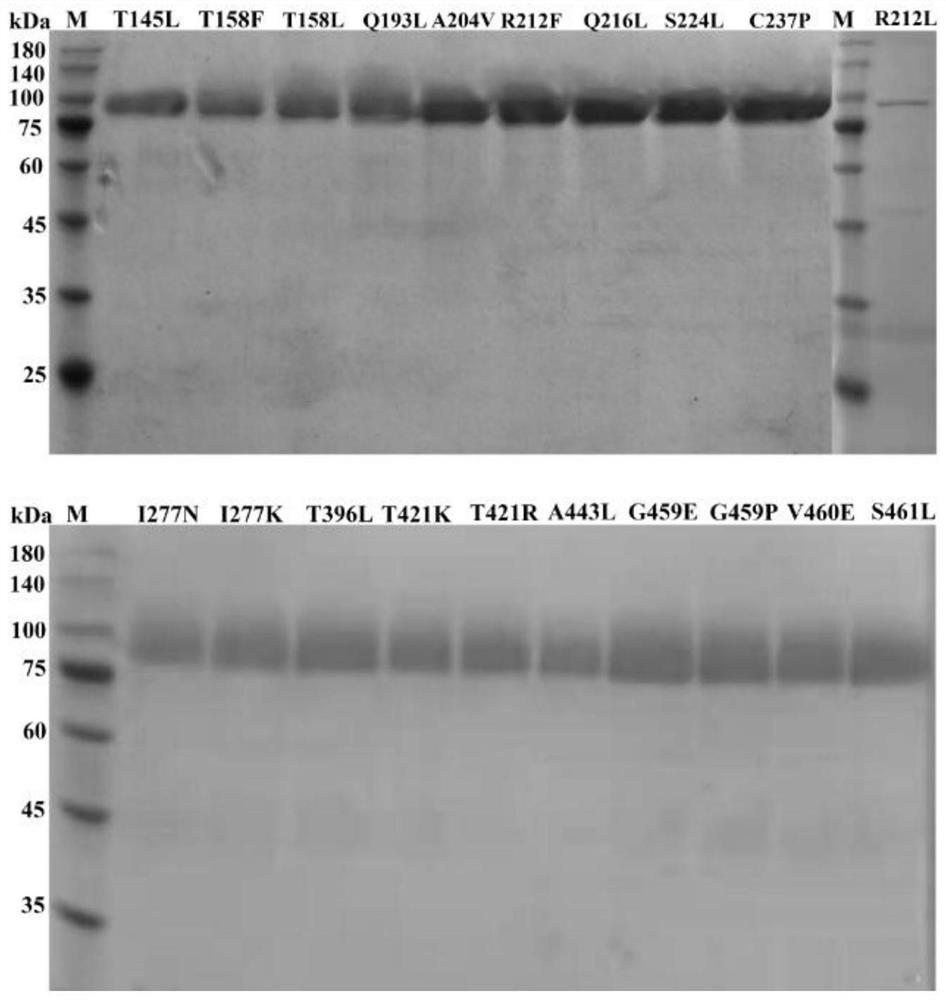
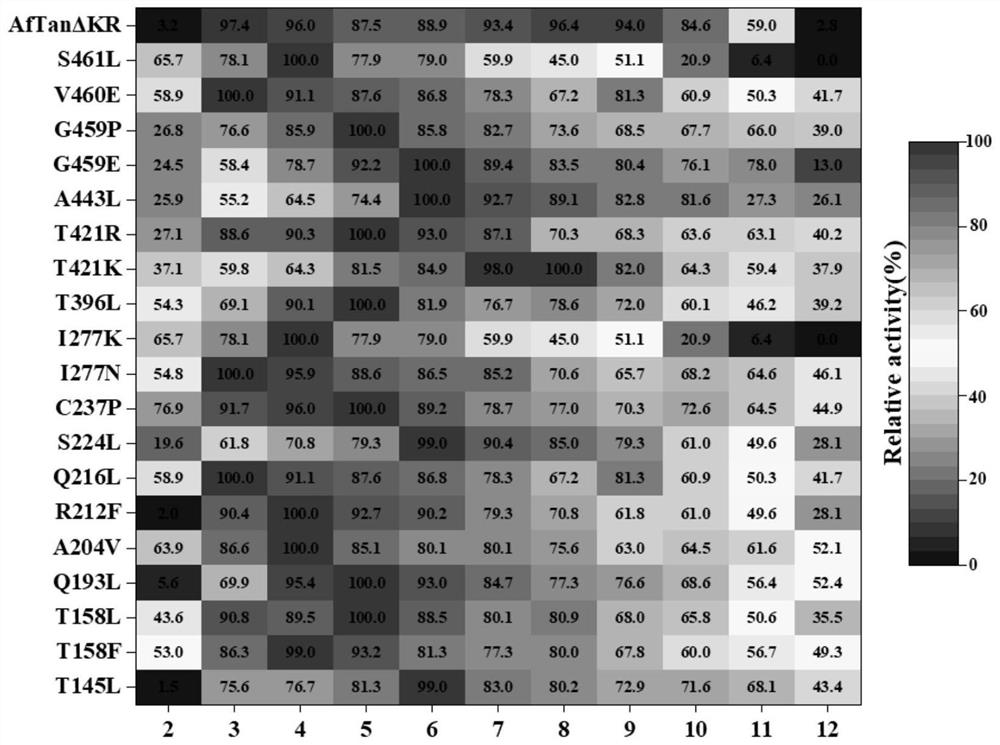
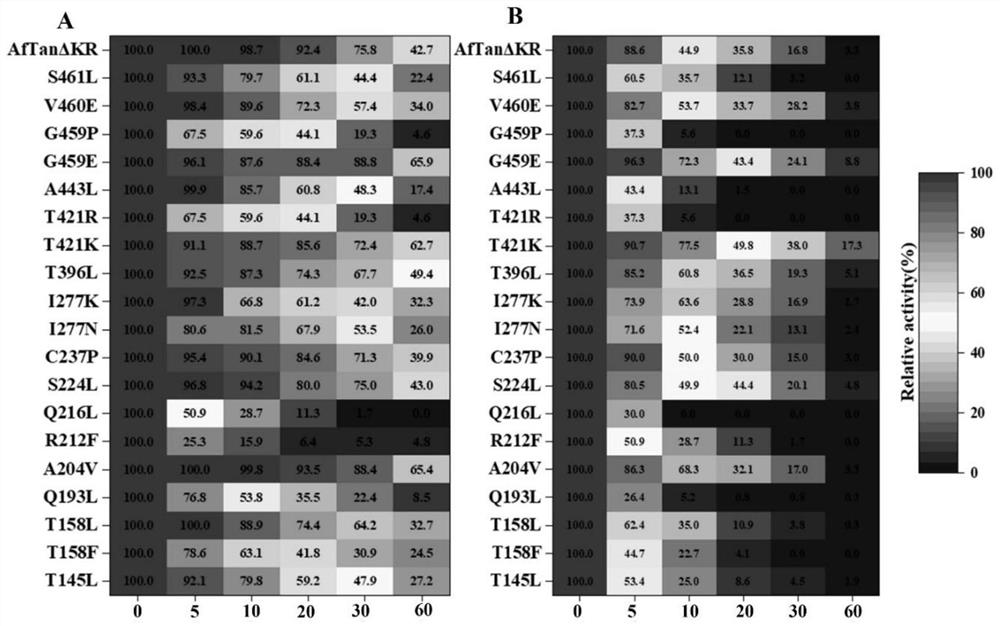
[0028] Example 3 SDS-PAGE analysis and activity identification of tannase thermostable mutants
[0029] SDS-PAGE analysis was carried out on the thermostable mutants of tannase. All 20 mutants were expressed in the recombinant strain of Pichia pastoris GS115, and the results were as follows: figure 1 shown. The apparent molecular weight of each mutant is between 75-100kDa, which is the same as that of AfTan2.0. When the tannase activity of each mutant was determined, it was found that the mutant R230L lost the tannase activity, and the remaining 19 mutants T163L, T176L, T176F, Q211L, A222V, R230F, Q234L, S242L, C255P, I295N, I295K, T412L, T437K, T437R, A459L, G475E, G475P, V476E and S477L still had tannase enzyme activity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Apparent molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com