Method for preparing stable porous amorphous calcium carbonate in alcohol solvent
An amorphous calcium carbonate, porous technology, applied in the field of materials, can solve problems such as unseen, and achieve the effects of low cost, low equipment requirements, and simple process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. Prepare 0.1mol / L calcium chloride ethylene glycol solution and 0.1mol / L potassium carbonate ethylene glycol solution;
[0031] 2. Mix the calcium chloride ethylene glycol solution and potassium carbonate ethylene glycol solution in step 1 at 20°C, stir for 2 minutes to fully react, and then age at 20°C for 1 hour;
[0032] 3. Centrifuge the suspension aged for 1 hour to obtain a precipitate. The precipitate is first treated with glycerin to remove the by-product potassium chloride, and then treated with ethanol to remove the glycerin on the surface, then centrifuged, and then vacuumed at room temperature After drying for 12 hours, a white powder was finally obtained, that is, porous amorphous CaCO3 ;
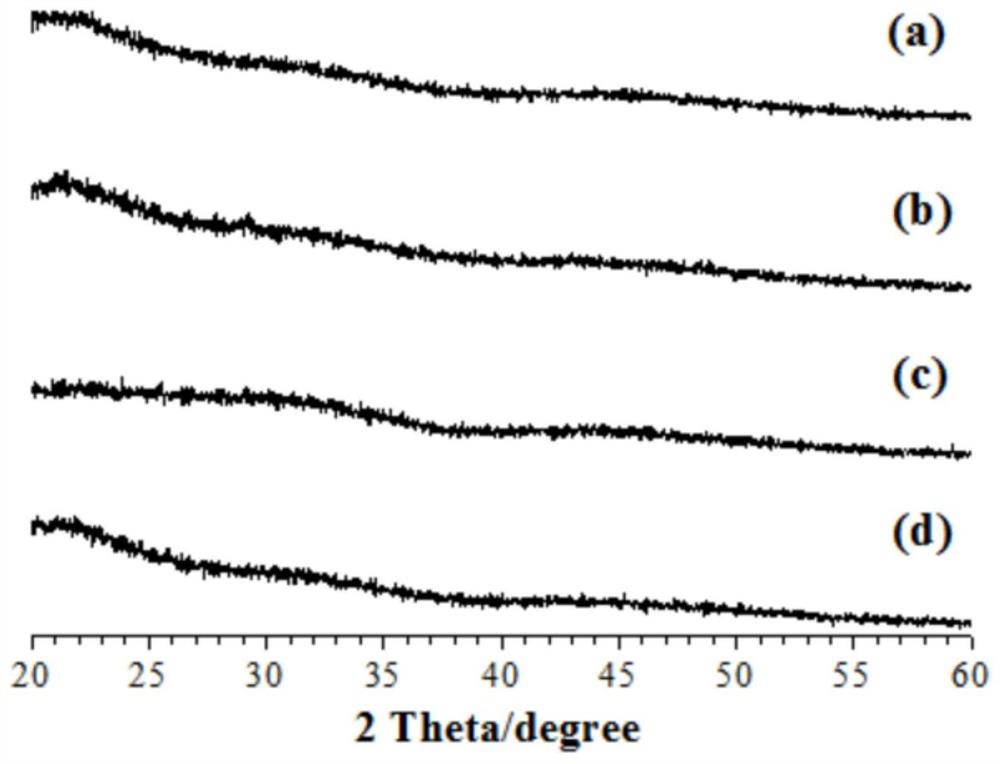
[0033] 4. Carry out XRD detection and SEM observation to the obtained product, the result is as follows figure 1 (a) and figure 2 , indicating that the obtained product is pure amorphous CaCO 3 , amorphous CaCO 3 Porous irregular particles.
Embodiment 2
[0035] 1. Prepare 0.4mol / L calcium chloride ethylene glycol solution and 0.4mol / L potassium carbonate ethylene glycol solution;
[0036] 2. Mix the calcium chloride ethylene glycol solution and potassium carbonate ethylene glycol solution in step 1 at 0°C, stir for 2 minutes to fully react, and then age at 0°C for 1 week;
[0037] 3. Centrifuge the suspension that has been aged for 1 week to obtain a precipitate. The precipitate is first treated with glycerin to remove the by-product potassium chloride, and then treated with ethanol to remove the glycerin on the surface, then centrifuged, and then vacuumed at room temperature After drying for 12 hours, a white powder was finally obtained, that is, porous amorphous CaCO 3 ;
[0038] 4. Carry out XRD detection to the obtained product, the result is as follows figure 1 (b), indicating that the obtained product is pure amorphous CaCO 3 .
Embodiment 3
[0040] 1. Prepare 0.4mol / L calcium chloride ethylene glycol solution and 0.4mol / L potassium carbonate ethylene glycol solution;
[0041] 2. Mix the calcium chloride ethylene glycol solution and potassium carbonate ethylene glycol solution in step 1 at 20°C, stir for 2 minutes to fully react, and then age at 20°C for 1 day;
[0042] 3. Centrifuge the suspension that has been aged for 1 day to obtain a precipitate. The precipitate is first treated with glycerin to remove the by-product potassium chloride, then treated with ethanol to remove the glycerin on the surface, and then centrifuged, and then heated at room temperature After vacuum drying for 12 hours, a white powder was finally obtained, that is, porous amorphous CaCO 3 ;
[0043] 4. Carry out XRD detection to the obtained product, the result is as follows figure 1 (c), showing that the obtained product is pure amorphous CaCO 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com