Novel method for synthesizing cholesterol from 21-hydroxy-20-methylpregna-4-ene-3-ketone as raw material
A synthetic method, the technology of methylpregnant, which is applied in the field of steroidal compound synthesis, can solve the problems of poor selective reduction, poor purification of products, high cost, etc., and achieves environmental protection process, high yield and low production cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
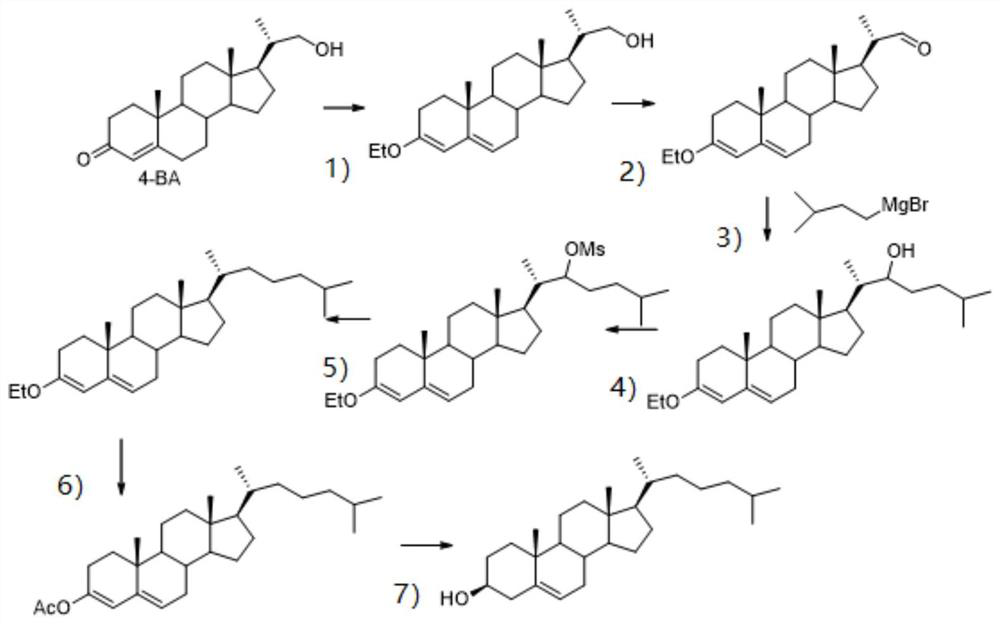
[0040] Cholesterol synthesis method, as attached figure 1 shown, including the following steps:
[0041] 1 Etherification reaction: In a 500ml three-necked flask, put 20g of 4-BA, 70ml of absolute ethanol, 120mg of phosphotungstic acid, stir, add 16ml of triethyl orthoformate, stir for 4 hours at room temperature, and pour 400ml of sodium bicarbonate solution after the reaction 21-hydroxy-20-methylpregna-3-ethoxy-3,5-diene 21g was obtained by filtering, beating with 80ml of petroleum ether, and drying under reduced pressure. The molar yield is 97%.
[0042] 2 Oxidation reaction: In a 500ml three-necked flask, put 21g of 21-hydroxy-20-methylpregna-3-ethoxy-3,5-diene, 100ml of DMSO, cool down to 0-10℃ in ice water, add triethylamine 56.2ml, add 32.8g sulfur trioxide pyridinium salt in batches, stir and react at room temperature for 3h, pour into 500ml ice water after reaction, extract with ethyl acetate, wash with water, wash with brine, dry and concentrate to obtain 3-ethoxy-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com