Medical application of iridoid glycoside compound
A compound and application technology, applied in the direction of medical preparations containing active ingredients, pharmaceutical formulations, applications, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
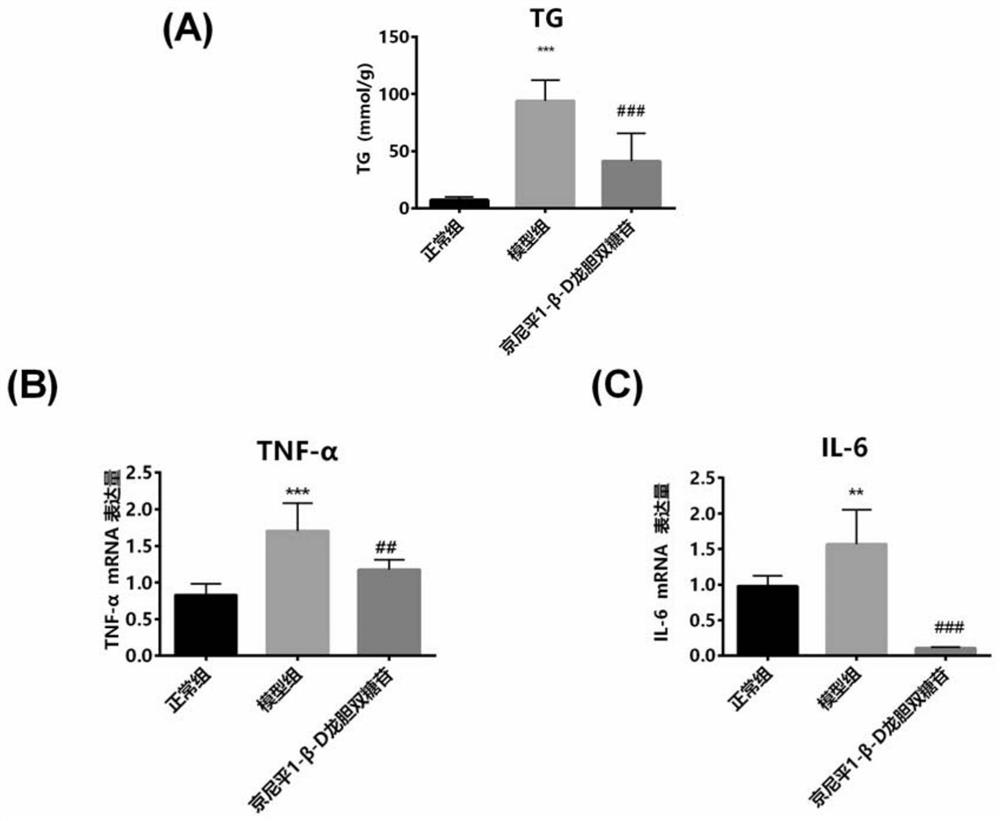
[0073] Genipin 1-β-D-gentiobioside anti-inflammatory and lipid-lowering experiments in vitro
[0074] 1. Experiments and methods
[0075] 1.1 Cell culture and administration method
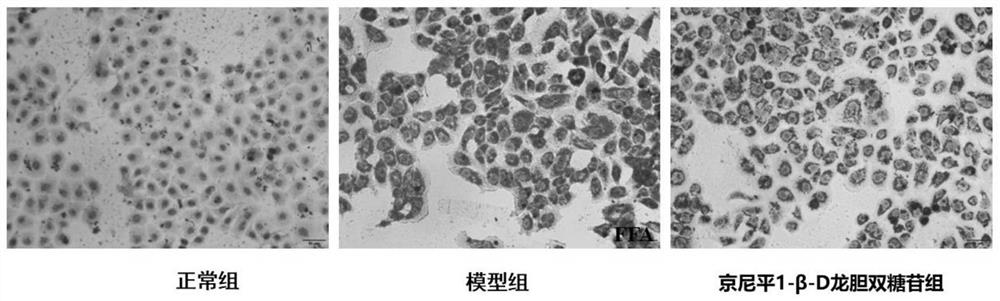
[0076] Human liver cancer cell line HepG2 was inoculated into 6-well plates (density about 1×10 5 / ml), placed at 37°C, 5% CO 2 , 95% humidity in an incubator for 24 hours, divided into normal control group, model group and genipin 1-β-D gentiobioside group, 4 wells in each group. The normal control group was given DMEM medium, and the model group added FFA (oleic acid 0.3mM: palmitic acid 0.15mM) in the DMEM medium; 0.3 mM: palmitic acid (0.15 mM) and genipin 1-β-D gentiobioside (100 μM) were co-incubated, and after 24 hours of incubation, the cells were collected.
[0077] 1.2 Determination of TG
[0078] Aspirate the 12-well plate culture medium, wash (0.5ml / well) with PBS, (200μl / well) trypsinize for 3min; dilute to 800μl / well with PBS, pipette each well, then draw the liquid into the EP ...
Embodiment 2
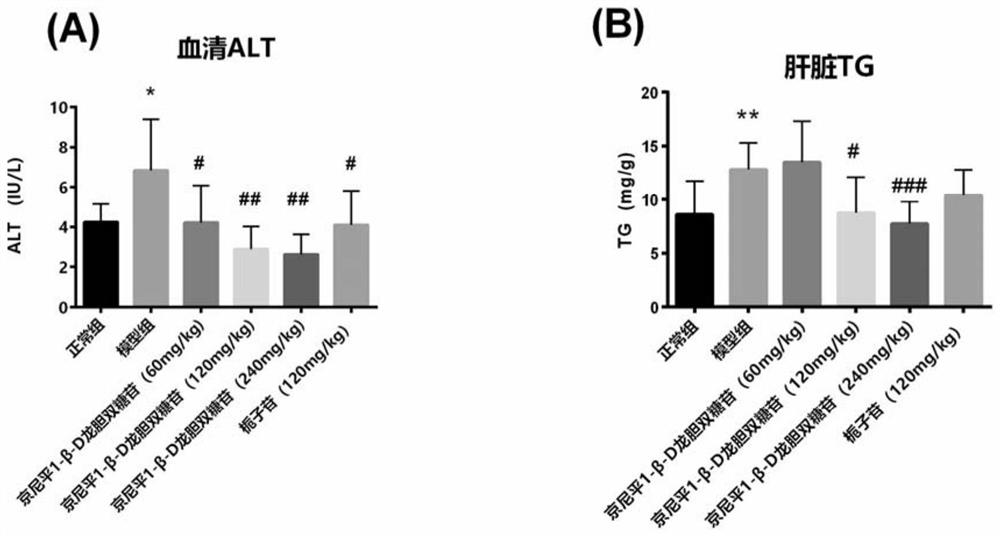
[0110] In vivo efficacy experiment of genipin 1-β-D gentiobioside and geniposide on non-alcoholic fatty liver disease
[0111] 1. Experimental method
[0112] 1.1. Experimental materials
[0113] Fifty male 4-week-old C57 mice were purchased from Shanghai Slack Experimental Animal Center, and were bred in the SPF feeding room of the Experimental Animal Center of Shanghai University of Traditional Chinese Medicine; the high-fat feed and its control feed were purchased from RESEARCH DIEATS Company, the item numbers are D12331 and D12328 , fructose and sucrose were purchased from Nantong Trofee Company, all with irradiation certificates. Genipin 1-β-D gentiobioside is isolated and purified from Gardenia jasmine by Shanghai Institute of Materia Medica, Chinese Academy of Sciences, and its purity is >98%. Dangfei Liganning was purchased from Sichuan Meidakang Pharmaceutical Co., Ltd., the batch number is Z51020085.
[0114] 1.2. Animal modeling, grouping and administration
[0...
Embodiment 3
[0130] Inhibition of NLRP3 by genipin 1-β-D-gentiobioside in vivo and in vitro
[0131] 1. Experiments and methods
[0132] (In vivo pre-test animals and modeling are the same as above)
[0133] 1.1 Cell culture and administration method
[0134] Mouse normal liver cell line AML-12 was inoculated into 6-well plate (density about 1×10 5 / ml), placed at 37°C, 5% CO 2 , in an incubator with 95% humidity for 24 hours, divided into normal control group (Con), model group (FFA) and genipin 1-β-D gentiobioside group (FFA+GG), 4 wells in each group . The normal control group was given DMEM medium, and the model group added FFA (oleic acid 0.3mM: palmitic acid 0.15mM) in the DMEM medium; 0.3 mM: palmitic acid (0.15 mM) and genipin 1-β-D gentiobioside (100 μM) were co-incubated, and after 24 hours of incubation, the cells were collected.
[0135] 1.2 RNA sample preparation and quality analysis
[0136] ① Discard the supernatant after the incubation, wash the cells twice with PBS,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com