Method for preparing crystal form ferric vanadate from vanadium-containing acid solution through pH slow release method
A technology of iron vanadate and vanadic acid, applied in chemical instruments and methods, iron compounds, inorganic chemistry, etc., can solve the problems of complex composition of iron vanadate, inability to realize the separation of iron vanadium, etc., and achieve easy industrialization promotion, easy control, Easy to filter and wash
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
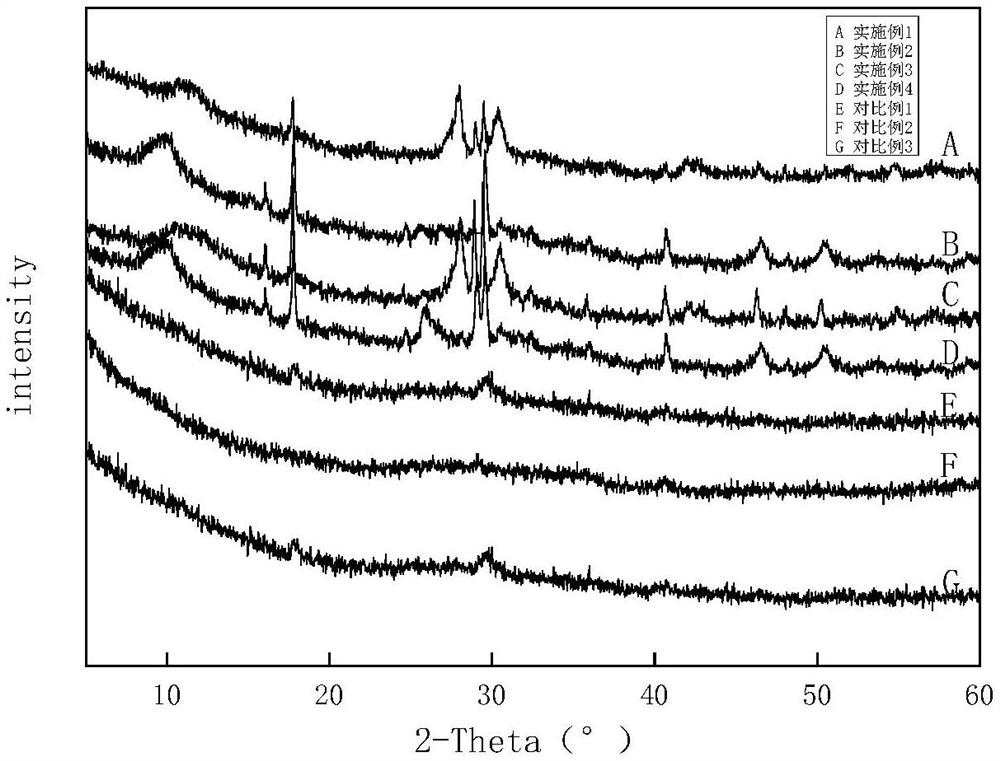
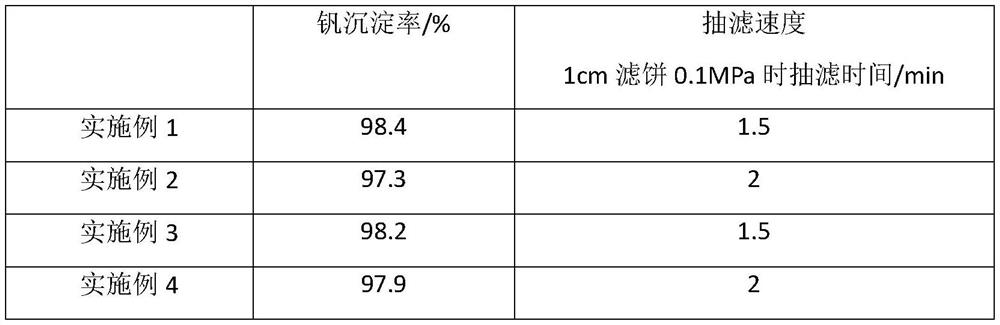
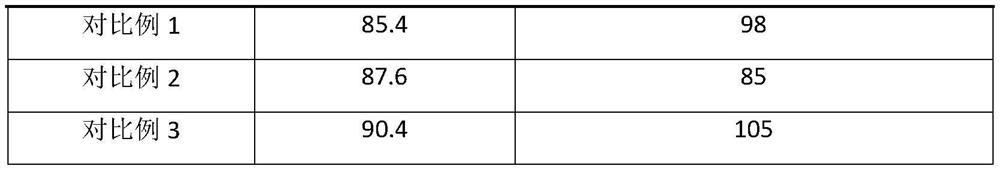
Image
Examples
Embodiment 1
[0048] This embodiment provides a method for preparing crystalline ferric vanadate by a pH slow-release method in a vanadium-containing acid solution, wherein the vanadium-containing acid solution is a stone coal acid leaching solution, wherein the main element concentration is V1.89g / L, Fe 6.97 g / L, Al 12g / L, described method comprises the steps:
[0049] (1) Control the temperature of the pickling solution at 40°C, put 100mL of petroleum coke gasification ash acid solution in the reactor, add sodium chlorate and stir for oxidation for 15 minutes, wherein the amount of sodium chlorate is 1.0 times the theoretical amount of oxidant, The resulting solution was yellow;
[0050] (2) Add sodium hydroxide solution to the above-mentioned oxidized solution to quickly adjust the pH to 1.4, and the adjustment time is 5 minutes;
[0051] (3) adding a crystal form control agent, 0.02% magnesium sulfate solution, to the above solution;
[0052] (4) Slowly adjust the pH to 2.5 with magne...
Embodiment 2
[0057] This embodiment provides a kind of method that contains vanadium acid solution pH sustained release method to prepare crystalline form ferric vanadate, described vanadium-containing acid solution is vanadium-containing tailings acid dipping solution, wherein main element concentration is V 2.45g / L, Fe 2.57g / L, Al 1g / L, described method comprises the steps:
[0058] (1) Control the temperature of the acid dipping solution at 20°C, put 100mL of petroleum coke gasification ash acid solution in the reactor, add sodium chlorate and stir for oxidation for 60 minutes, wherein the amount of sodium chlorate is 1.1 times the theoretical amount of oxidant, The resulting solution was yellow;
[0059] (2) Add sodium hydroxide solution to the above-mentioned oxidized solution to quickly adjust the pH to 1.0, and the adjustment time is 25 minutes;
[0060] (3) Add crystal form control agent, 0.05% magnesium chloride solution to the above solution;
[0061] (4) Slowly adjust the pH t...
Embodiment 3
[0066] The present embodiment provides a kind of method that contains vanadium acid liquid pH sustained release method to prepare crystalline form ferric vanadate, described containing vanadium acid liquid is petroleum coke hydrogen ash slag pickling liquid, and wherein main element concentration is V 3.25g / L, Fe 8.31g / L, Al 10g / L, described method comprises the steps:
[0067] (1) Control the temperature of the acid dipping solution at 50°C, put 100mL of petroleum coke gasification ash acid solution in the reactor, add sodium chlorate and stir for oxidation for 10 minutes, wherein the amount of sodium chlorate is 1.2 times the theoretical amount of oxidant, The resulting solution was yellow;
[0068] (2) Add sodium hydroxide solution to the above-mentioned oxidized solution to quickly adjust the pH to 1.6, and the adjustment time is 20 minutes;
[0069] (3) Add crystal form control agent, 0.04% magnesium nitrate solution to the above solution;
[0070] (4) Slowly adjust th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com