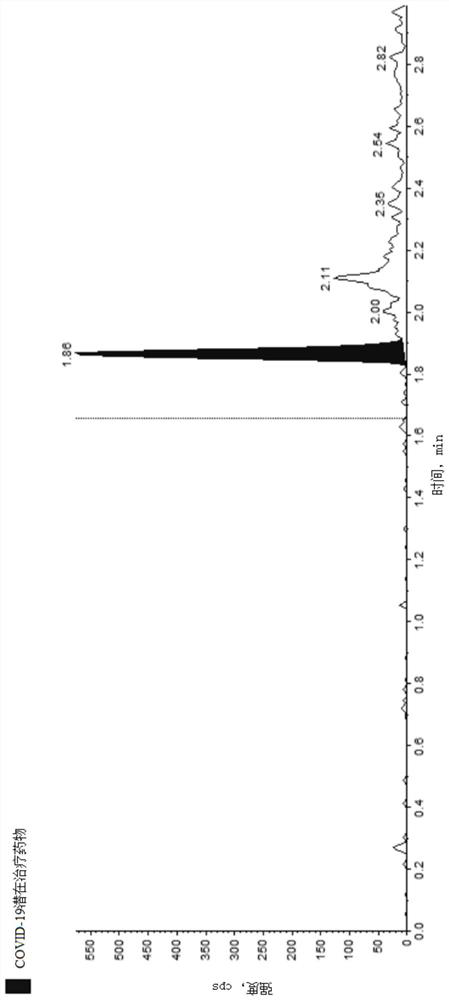
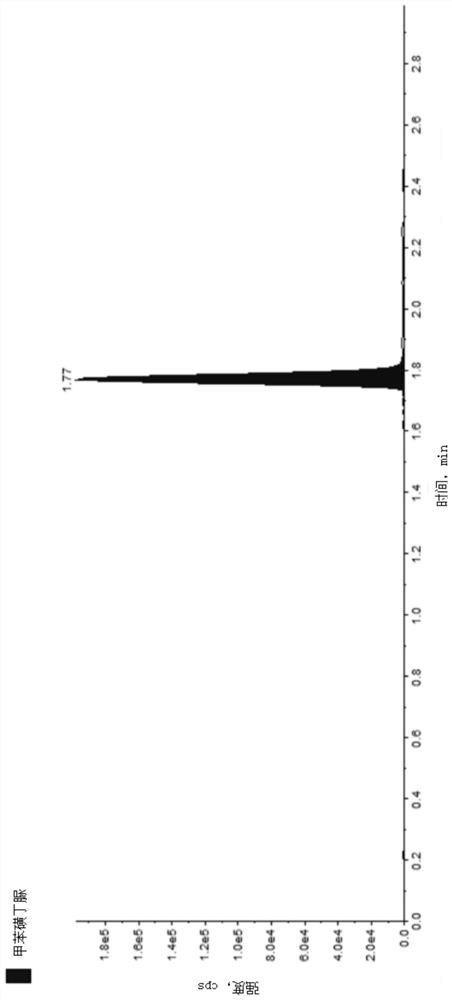
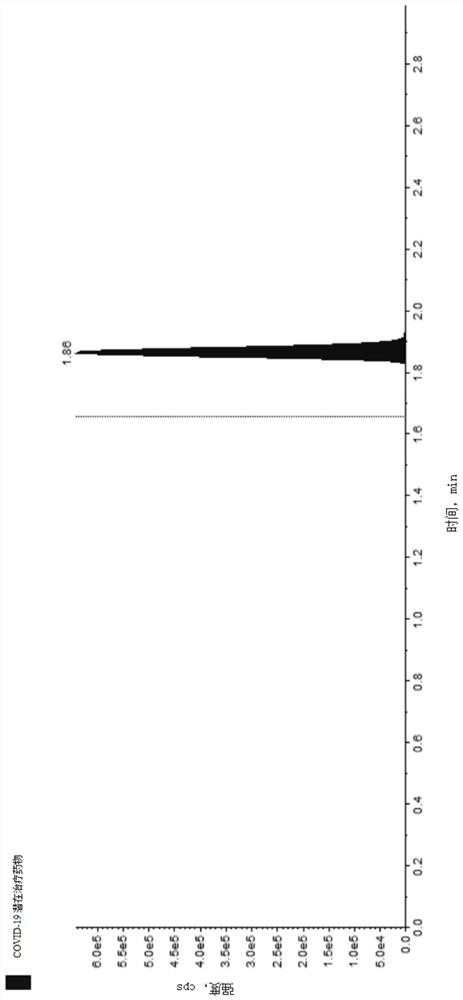
Method for detecting COVID-19 potential therapeutic drug in macaca fascicularis plasma
A technology for COVID-19 and therapeutic drugs, applied in measurement devices, instruments, scientific instruments, etc., can solve problems such as pending clinical verification and imminent research and development of anti-new coronavirus drugs, and achieve the effects of short analysis time, high sensitivity, and linear accuracy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] A method for detecting potential therapeutic drugs for COVID-19 in cynomolgus monkey plasma, comprising:
[0056] 1. Preparation of reagents
[0057] 1.1 Mobile phase A (MPA): formic acid (FA) aqueous solution with a volume concentration of 0.1%.
[0058] Take 1000mL H 2 Add 1mL FA to O in the solvent bottle and mix well. Store at room temperature, valid for 1 month.
[0059] 1.2 Mobile phase B (MPB): MeOH (methanol)
[0060] Take 1000mL MeOH in a solvent bottle. Store at room temperature, valid for 3 months.
[0061] 1.3 Strong washing solution (SNW): MeOH:ACN:IPA:DMSO=1:1:1:1, v / v / v / v.
[0062] Take 500mL MeOH, 500mL ACN (acetonitrile), 500mL IPA (isopropanol), 500mL DMSO (dimethylsulfoxide), and mix well. Store at room temperature, valid for 3 months.
[0063] 1.4 Weak wash solution (WNW): MeOH:H 2 O=1:1, v / v.
[0064] Take 1000mLMeOH, 1000mL H 2 O, mix well. Store at room temperature, valid for 3 months.
[0065] 1.5 Precipitating agent & internal stand...
PUM
| Property | Measurement | Unit |
|---|---|---|
| percent by volume | aaaaa | aaaaa |
| percent by volume | aaaaa | aaaaa |
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com