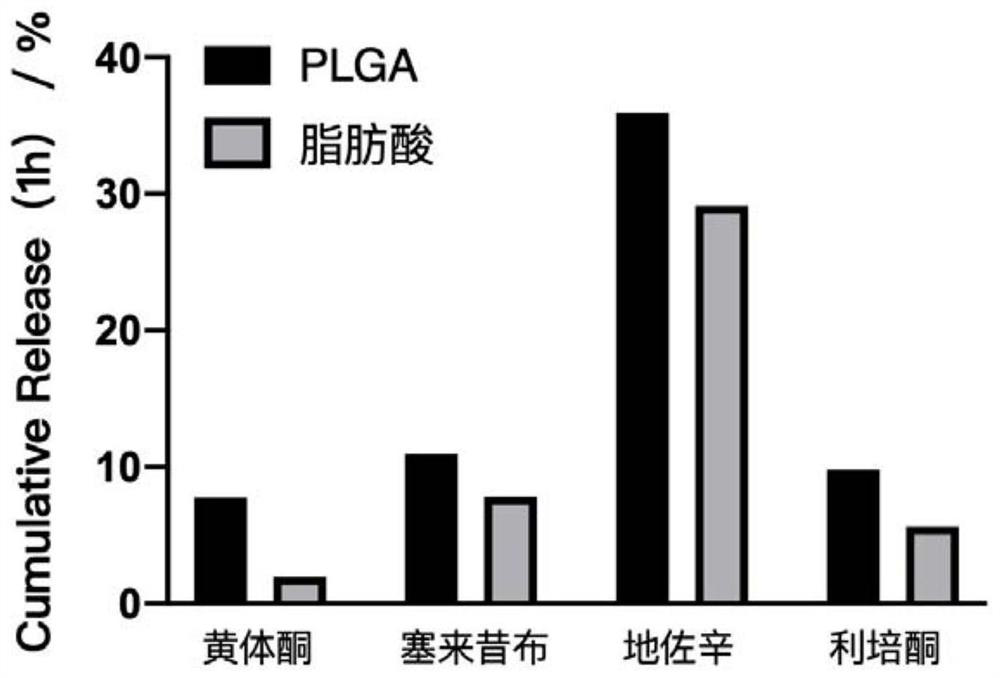
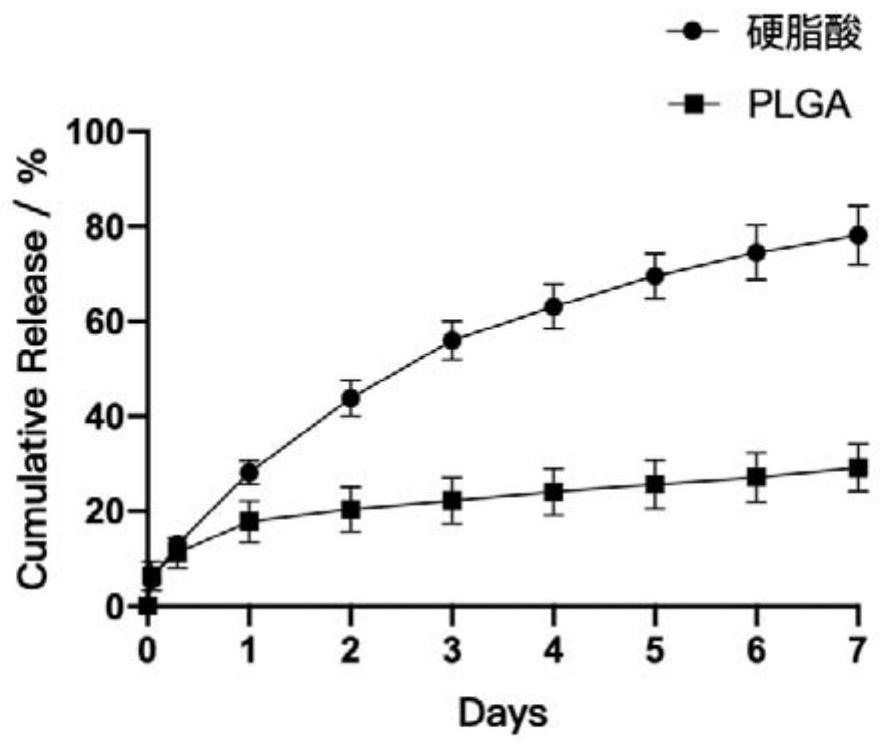
Fatty acid sustained-release composition for injection and preparation method and application thereof
A sustained-release composition and fatty acid technology, applied in the field of medicine, can solve problems affecting the stability of drug release of long-acting preparations, inability to adapt to clinical drug administration, unfavorable product quality control, etc., to improve burst release phenomenon, solid gel Dense structure, effect of promoting drug absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Take 100 mg of progesterone, dissolve it in 700 mg of NMP, filter through a 0.22 μm microporous membrane to obtain a drug solution, then add 200 mg of stearic acid under sterile conditions, and stir magnetically for about 1 hour until completely dissolved , to obtain a clear and transparent liquid, sub-packaged, sealed, that is.
Embodiment 2
[0028] Take 100 mg of progesterone, dissolve it in 700 mg of NMP, filter through a 0.22 μm microporous membrane to obtain a drug solution, then add 200 mg of PLGA under sterile conditions, and stir magnetically for about 1 hour under sterile conditions until it is completely dissolved and clarified Transparent liquid, packaged and sealed, ready to use.
Embodiment 3
[0030] Take 100 mg of ethinyl estradiol, dissolve it in 800 mg of NMP, filter it through a 0.22 μm microporous membrane to obtain a drug solution, then add 200 mg of arachidic acid under sterile conditions, and stir magnetically for about 1 hour under sterile conditions until it is completely dissolved , to obtain a clear and transparent liquid, sub-packaged, sealed, that is.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com