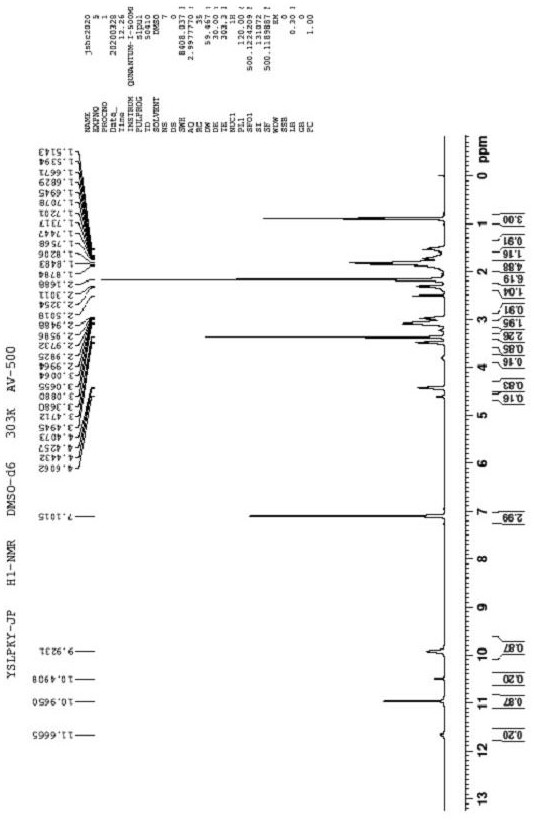
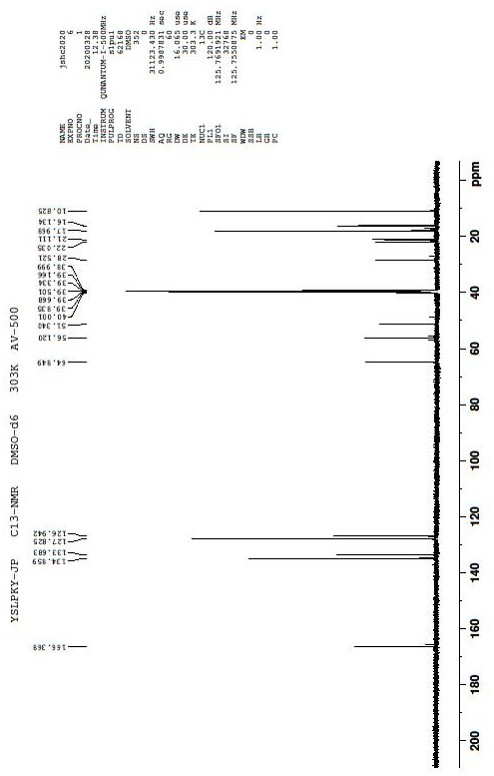
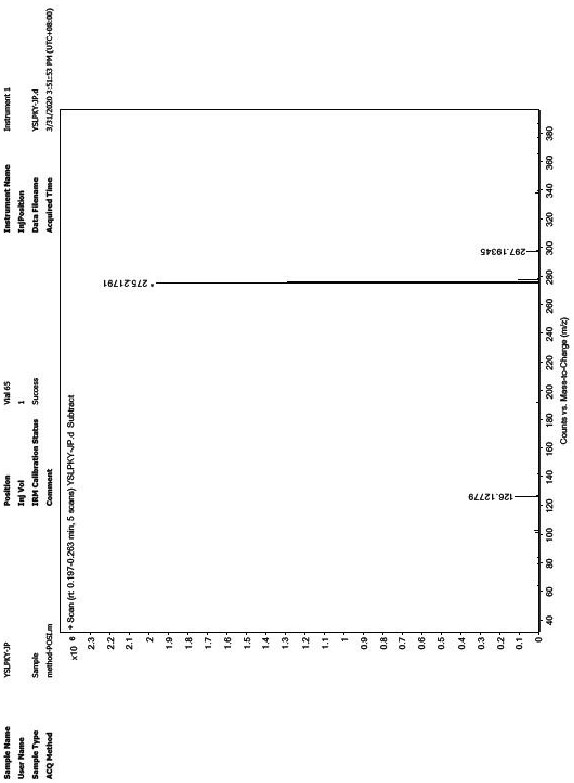
Preparation method of ropivacaine hydrochloride
A technology of ropivacaine hydrochloride and hydrochloric acid, applied in the field of chemistry, can solve problems such as being unfavorable for industrialized production, unsuitable for industrialized production, poor salt-forming effect, etc., avoiding protection and deprotection, and inhibiting the generation of quaternary ammonium salt impurities , easy-to-control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1 prepares ropivacaine hydrochloride
[0059] Step 1: Preparation of Intermediate 1
[0060] Add (S)-piperidine-2-carboxylic acid (100.00g, 0.77mol) in the 2000mL reaction flask, propionaldehyde (104.32g, 2.32mol) and dichloromethane (800mL), nitrogen replaces the air in the reaction flask, and then Add sodium cyanoborohydride (111.90g, 2.30mol) and react at 35°C for 10 hours. After the reaction is completed, quench with saturated aqueous sodium bicarbonate (500mL), separate the organic phase, and wash the aqueous phase with dichloromethane (500mL*2) extraction, combined organic phases, dried over anhydrous magnesium sulfate, concentrated under reduced pressure at 40°C-45°C to obtain 125g of intermediate 1, yield 94.3%.
[0061] Step 2: Preparation of Intermediate 2
[0062] Add intermediate 1 (100.00g, 0.58mol), dichloromethane (600mL) and N,N-dimethylformamide (2mL) into a 2000mL reaction flask, cool down to an internal temperature of 5°C in an ice-water ...
Embodiment 2
[0071] Embodiment 2 prepares ropivacaine hydrochloride
[0072] Step 1: Preparation of Intermediate 1
[0073] Add (S)-piperidine-2-carboxylic acid (100.00g, 0.77mol) in the 2000mL reaction flask, propionaldehyde (104.32g, 2.32mol) and dichloromethane (800mL), nitrogen replaces the air in the reaction flask, and then Add sodium acetate borohydride (380.69g, 2.32mol) and react at 35°C for 10 hours. After the reaction, quench with saturated aqueous sodium bicarbonate (500mL), separate the organic phase, and wash the aqueous phase with dichloromethane (500mL *2) extraction, combined organic phases, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure at 40°C to 45°C to obtain 110g of intermediate 1 with a yield of 83.0%.
[0074] Step 2: Preparation of Intermediate 2
[0075] Add Intermediate 1 (100.00g, 0.58mol), dichloromethane (600mL) and N,N-dimethylformamide (2mL) into a 2000mL reaction flask, cool down to an internal temperature of 10°C in an ic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com