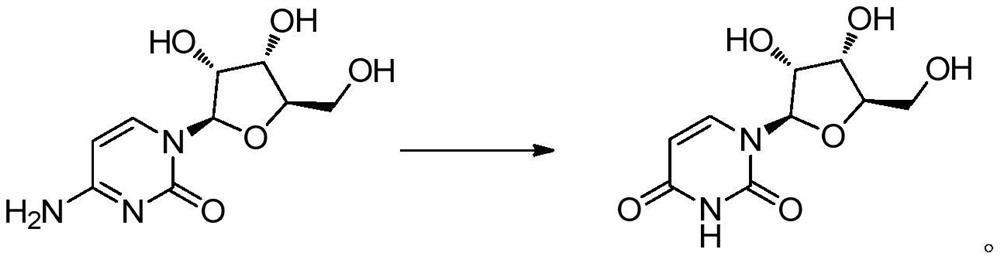
Synthesis process of uridine
A technology of uridine and cytidine, which is applied in the field of uridine synthesis technology, can solve the problems of complex process, many pollutants, and less output, and achieve the effects of simple synthesis process, high synthesis conversion rate and convenient post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
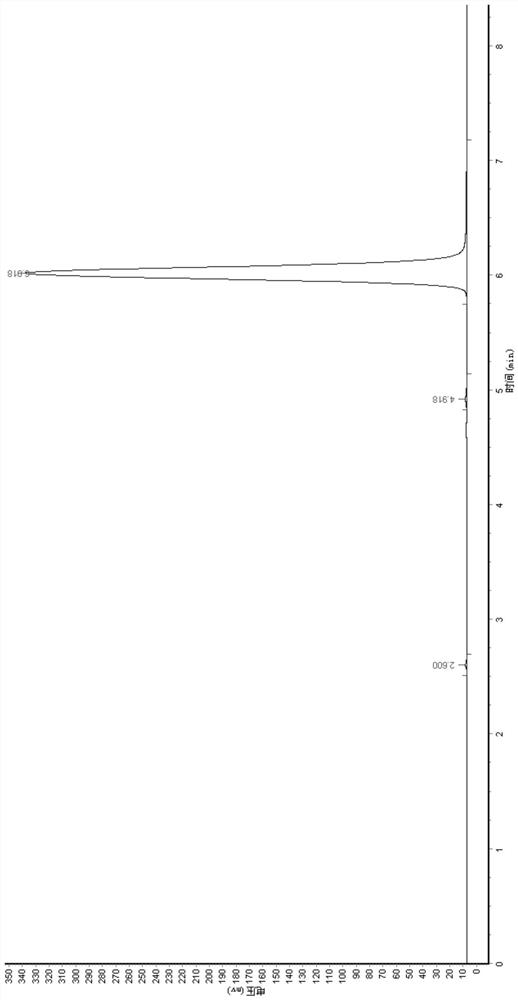
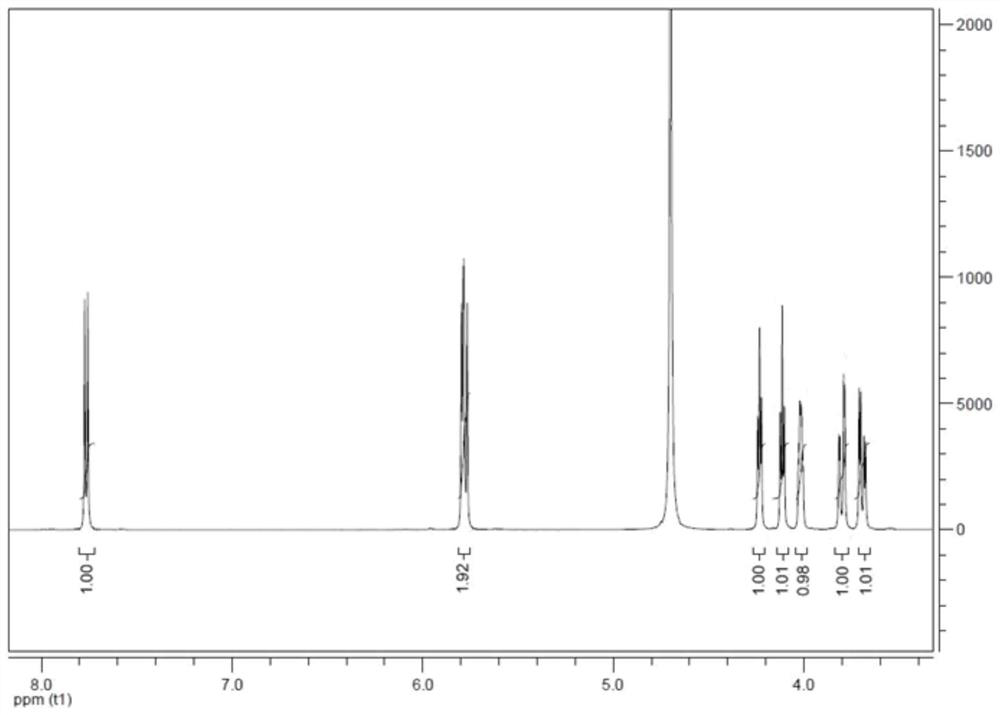
[0035] First add 100g of water to the reaction bottle, control the temperature at 15°C, then mix and dissolve 100g (0.411mol) of cytidine nucleoside and 150g (1.233mol) of 30wt% hydrochloric acid to prepare 250g of cytidine hydrochloride solution; 250g (0.906mol) of 25wt% sodium nitrite solution, 250g of cytidine hydrochloride solution and 250g of 25% sodium nitrite solution were added dropwise to the reaction flask at the same time, stirred and reacted, and the pH of the reaction was controlled to be 3.5-4.0, and the duration of the addition was Both were 3 hours, and the dropping temperature was 15°C. After 3 hours, after the dropping was completed, the reaction was incubated for 5 hours, and the residue of the raw material was detected by HPLC, and the reaction was completed, and the reaction conversion rate reached 98.5%.
[0036] After the reaction is completed, adjust the pH of the reaction solution to 2.0, raise the temperature to 60° C., concentrate under reduced pressu...
Embodiment 2
[0040] First add 100g of water to the reaction bottle, control the temperature at 15°C, then mix and dissolve 100g (0.411mol) of cytidine nucleoside and 150g (1.233mol) of 30wt% hydrochloric acid to prepare 250g of cytidine hydrochloride solution; 250g (0.906mol) of 25wt% sodium nitrite solution, 250g of cytidine hydrochloride solution and 250g of 25% sodium nitrite solution were added dropwise to the reaction flask at the same time, stirred and reacted, and the pH of the reaction was controlled to be 3.5-4.0, and the duration of the addition was Both were 3 hours, and the dropping temperature was 15° C. After 3 hours, after the dropping was completed, the reaction was incubated for 5 hours, and the residual raw materials were detected by HPLC, and the reaction was completed, and the reaction conversion rate was 98.7%.
[0041] After the reaction is completed, adjust the pH of the reaction solution to 2.0, raise the temperature to 60°C, concentrate under reduced pressure for 30...
Embodiment 3
[0043]First add 100g of water to the reaction bottle, control the temperature at 15°C, then mix and dissolve 100g (0.411mol) of cytidine nucleoside (0.411mol) and 74g (1.233mol) of acetic acid to prepare 174g of cytidine acetate solution; another 25wt% 250g (0.906mol) of sodium nitrite solution, 174g of cytidine acetate solution and 250g of 25% sodium nitrite solution were stirred and reacted, and the pH of the reaction was controlled to be 4.0 to 4.5. The adding time was 3 hours, and the dropping temperature was 15 ℃, 3 hours later, after the dropwise addition was completed, the reaction was incubated for 7 hours, and the residue of the raw material was detected by HPLC and disappeared, and the reaction was completed, and the reaction conversion rate was 96.1%.
[0044] After the reaction is completed, adjust the pH of the reaction solution to 2.0, raise the temperature to 60°C, concentrate under reduced pressure for 30 minutes, adjust the pH to 7.0, continue to concentrate to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com