Fused-ring polymer donor material based on benzothiadiazole or benzoselenadiazole and preparation method of fused-ring polymer
A technology of benzothiadiazole and benzoselenodiazole, which is applied in the field of conjugated polymers of benzothiadiazole units and its preparation, to achieve strong light absorption range, low HOMO energy level, and high atom economy Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1. Preparation of conjugated polymers based on benzothiadiazole and benzoselenodiazole units
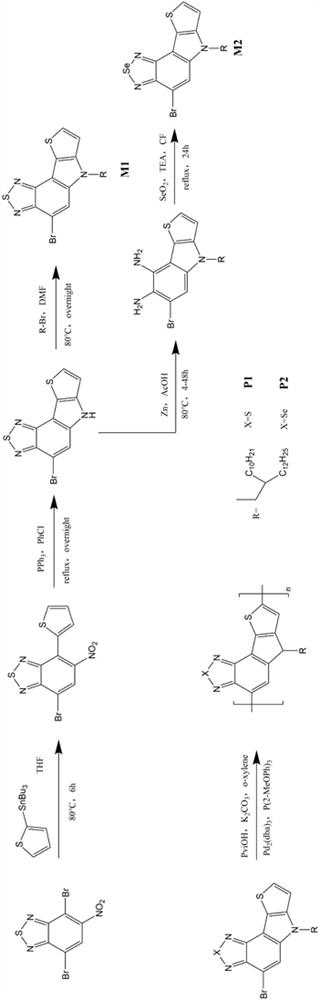
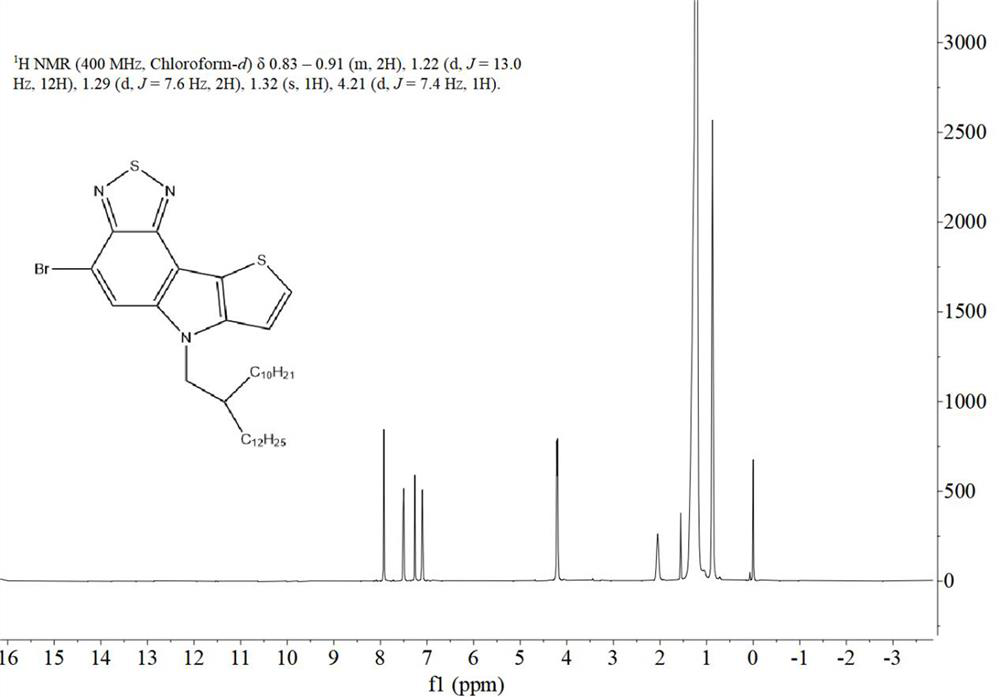
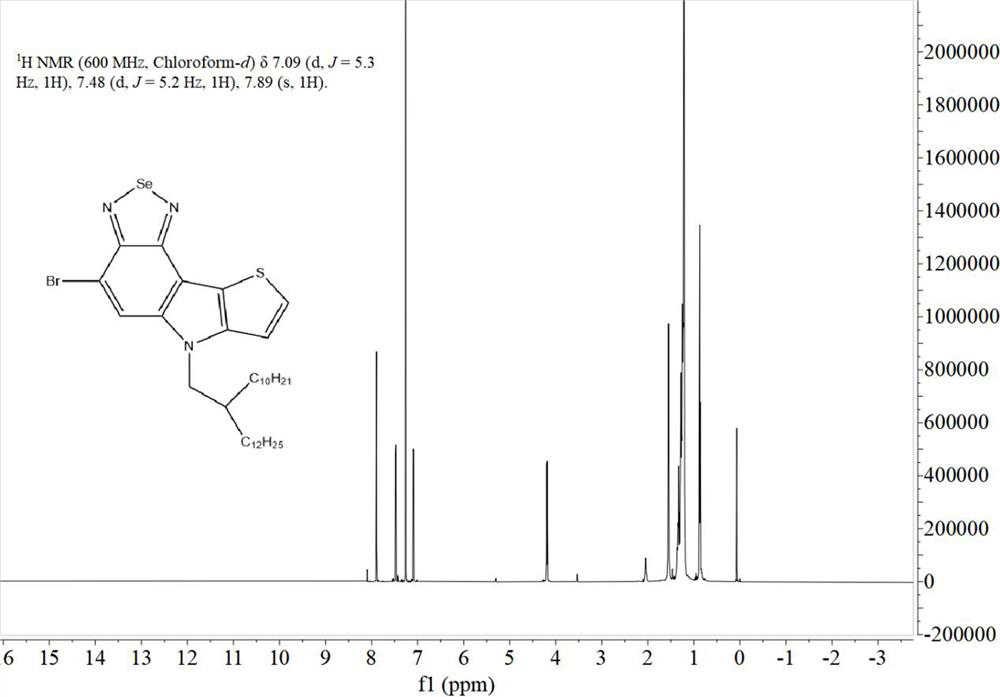
[0052] This example provides two soluble conjugated polymers based on benzothiadiazole or benzoselenodiazole units, the structural formulas of which are shown in Table 1, and their synthetic routes can be found in figure 1 .
[0053] Table 1
[0054]
[0055] 1.1. Preparation of monomer M1
[0056] The synthetic route of the first monomer M1 of the two polymers is exactly the same, which specifically includes the following steps:
[0057] Synthesis of a, 7-bromo-5-nitro-4-(thiophen-2-yl)benzo[c][1,2,5]thiadiazole:
[0058] Under argon conditions, 4,7-dibromo-5-nitrobenzo[c][1,2,5]thiadiazole (i.e. starting material A, synthetic reference patent literature: CN109879870A based on benzothiadiazole Synthesis and application of new functional materials of oxadiazole) (3.3896g, 10mmol), 2-(tributyltin-based) thiophene (3.7319g, 10mmol) and 50mL of dry THF were added t...
Embodiment 2
[0076] The molecular weight of embodiment 2, polymer P1 and P2
[0077] Adopt gel permeation chromatography to test the molecular weight of two kinds of donor materials: the number average molecular weight (M) of P1 and P2 n ) are 103.8kDa, 56.2kDa respectively, weight average molecular weight (M w ) were 359.8kDa and 310.0kDa, respectively, and the polydispersity index PDI were 3.46 and 5.51, respectively.
Embodiment 3
[0078] The thermogravimetric curve of embodiment 3, polymer P1 and P2
[0079] From the TGA curves, it can be seen that under an inert atmosphere, the decomposition temperatures corresponding to 5% mass loss of polymers P1 and P2 reach 435°C and 350°C respectively, indicating that they have good thermal stability, showing that the two can be used as donor materials Applications in organic solar cell devices and other optoelectronic device requirements.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com