Fringed pink formula granule quality control method
A quality control method and technology for formula granules, which are applied in measuring devices, instruments, scientific instruments, etc., can solve problems such as the influence of the quality of Qumai formula granules, and achieve the effects of improving the yield of paste, increasing the amount of leaching, and ensuring stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The invention discloses a quality control method of Qumai formula granules, which uses different batches of Qumai decoction pieces to prepare Qumai formula granules. The preparation process is as follows:
[0039] Take an appropriate amount of Qumai decoction pieces, decoct twice, add 15 times of water to the first decoction, boil for 1 hour, add 13 times of water for the second decoction, boil for 1 hour, filter, and concentrate the filtrate under reduced pressure at 60°C to a relative density of 1.00-1.05 pure extract, spray-dried with 10% dextrin, or 1% SiO 2Spray drying, or belt drying with 0% excipients into fine powder, adding appropriate amount of dextrin, dry granulation, packaging specifications are 1g / bag composite film, 100g / bottle, 250g / bottle, sealed storage.
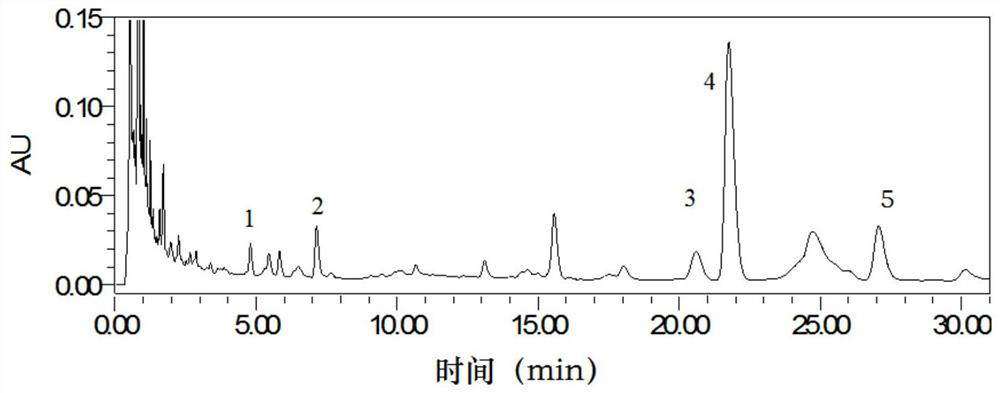
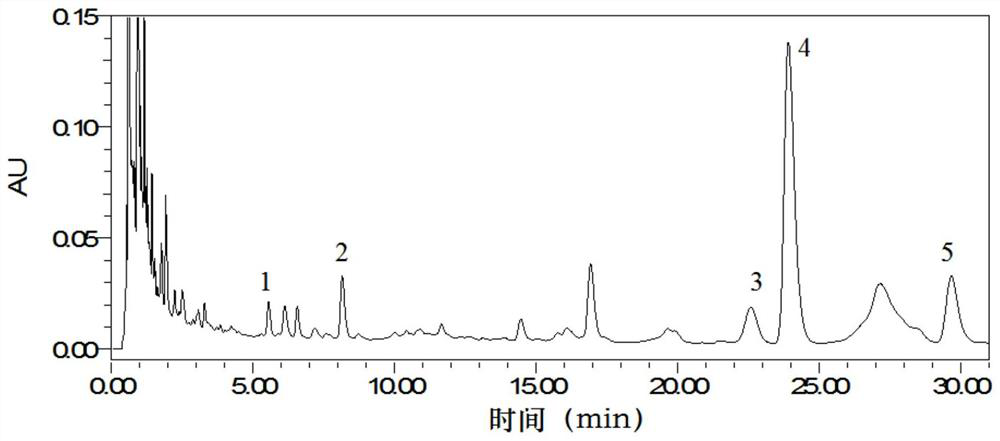
[0040] 1. Calculate the ratio of the peak area of the specified value of 1.06 to the peak area of the reference peak S and the similarity of the characteristic spectrum of Qumai formula granules...
Embodiment 2
[0073] In this embodiment, conventional methods are used to verify the precision, repeatability and stability of the characteristic map, as follows:
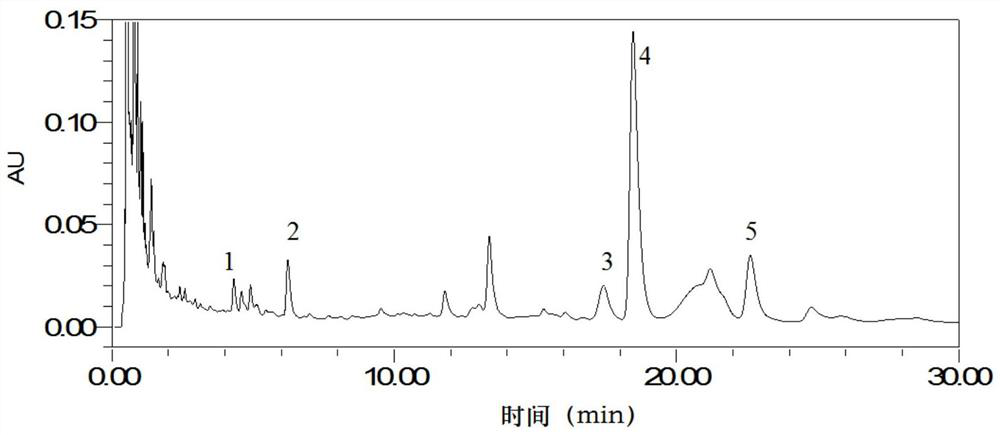
[0074] Take 6 parts of the test solution, obtain its characteristic spectrum, calculate the similarity with the reference material of the control medicinal material, and calculate the RSD. Results The similarities between the characteristic spectra of the 6 test product solutions and the reference materials of the control medicinal materials were between 0.850 and 0.851, both were greater than 0.80, and the RSD was 0.1%, which indicated that the characteristic spectra had good repeatability.
[0075] Using Waters UPLC H-Class, PDA detector, take the test solution, obtain its characteristic spectrum, calculate the similarity with the control medicinal material reference substance, and calculate the RSD. Results Among the characteristic maps obtained by Waters UPLC H-Class and PDA detector, the similarity between each characterist...
Embodiment 3
[0078] This embodiment provides the process of verifying the accuracy, intermediate precision, repeatability and stability of the total flavonoid content detection method, as follows:
[0079] Accuracy: Accurately weigh 10.558mg of rutin reference substance, put it in a 500ml volumetric flask, add 50% ethanol to set the scale, shake well, and set aside. Take a sample with a known content under the repeatability item, grind it finely, take about 0.05g, and accurately weigh it, a total of six parts, accurately add 50ml of rutin reference solution (19.553μg / ml) to each sample, and then accurately add 50 % ethanol 50ml, measure the absorbance of solution by above-mentioned preferred method, and calculate the content of total flavonoids. Results The measured recoveries ranged from 92.50% to 97.62%, the average recovery rate was 95.1%, and the RSD value was 2.0%, which met the recovery requirements of methodological verification, indicating that the results measured by this method w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com