Synthesis method of 4H-1, 2, 4-benzothiadiazine-1, 1-dioxide derivative
A technology of benzothiadiazine and 4H-1, applied in the field of synthesis of 4H-1,2,4-benzothiadiazine-1,1-dioxide derivatives, can solve the toxicity of ethyl orthoformate Large, difficult separation and purification, low yield of aldehyde ring closure, etc., to achieve the effect of reducing synthesis cost, high synthesis efficiency, and good functional group tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062]
[0063] Add N,N-diphenylmethacrylamide 1a (47 mg, 0.2 mmol), 1 mL of anhydrous dichloroethane, and then add chlorosulfonyl isocyanate (70 mg, 0.5 mmol) into a 10 mL dry reaction tube ), and the mixture was stirred at 80 °C for 5 h. After the reaction mixture was concentrated under reduced pressure, it was separated by silica gel column chromatography (eluent was petroleum ether / ethyl acetate (v / v = 5 / 1-1 / 2)) to obtain 54 mg of product 4-phenyl-3 -Isopropenyl-4H-1,2,4-benzothiadiazine-1,1-dioxide 2a, 90% yield.
[0064] This reaction also can be carried out in isonitrile, methylene dichloride, chloroform, toluene, and its corresponding reaction time and productive rate are as shown in table 1:
[0065] Table 1
[0066]
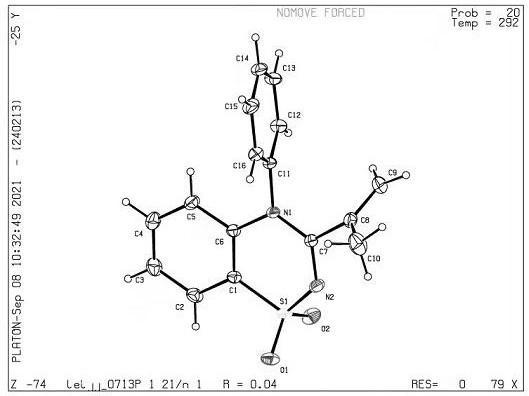
[0067] X-ray single crystal diffraction test is carried out to product 2a, can find that this product has the structural unit of benzothiadiazine (such as figure 1 shown).
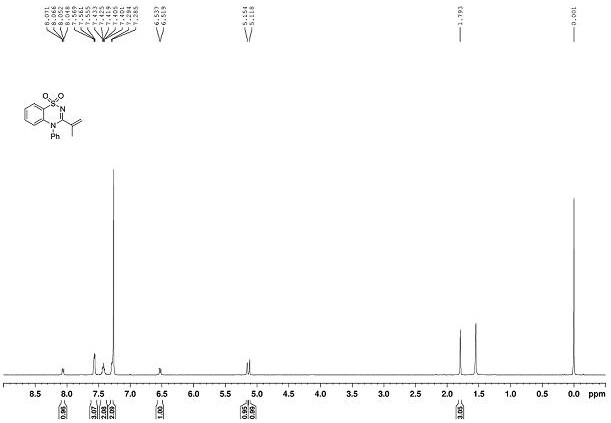
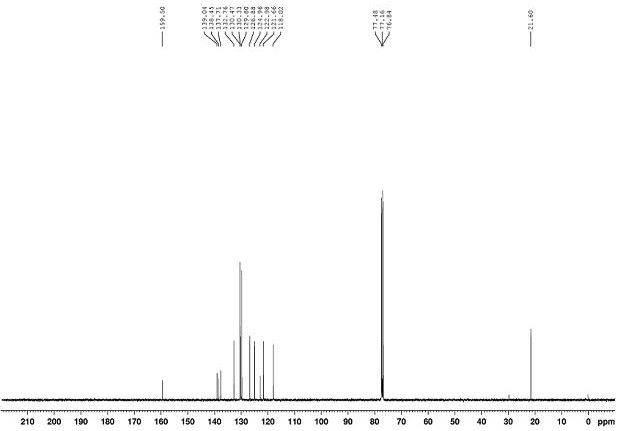
[0068] The NMR data of product 2a as Figure 2~3 As shown, the deta...
Embodiment 2
[0073]
[0074] Add N-methyl-N-phenylbenzamide 1b (42 mg, 0.2 mmol) to a 10 mL dry reaction tube, 1 mL of anhydrous dichloroethane, and then add chlorosulfonyl isocyanate (70 mg, 0.5 mmol), and the mixture was stirred at 80 °C for 6 h. After the reaction mixture was concentrated under reduced pressure, it was separated by silica gel column chromatography (eluent was petroleum ether / ethyl acetate (v / v = 5 / 1-1 / 2)) to obtain 49 mg of product 4-methyl-3 -Phenyl-4 H -1,2,4-Benzothiadiazine-1,1-dioxide 2b in 91% yield.
[0075] NMR data on product 2b as Figure 4~5 As shown, the details are as follows:
[0076] 1 H NMR (400 MHz, CDCl 3 ): δ 8.03 (dd, J = 1.2 Hz, 8.0 Hz, 1H), 7.71 (t, J = 7.2 Hz, 1H), 7.63 (d, J = 6.8 Hz, 2H), 7.47–7.58 (m, 4H), 7.42 (d, J =8.4Hz, 1H), 3.59(s, 3H).
[0077] 13 C NMR (100 MHz, CDCl 3 ): δ 160.8, 138.7, 133.4, 133.3, 131.9, 129.3, 128.9, 126.9, 124.8, 123.9, 116.4, 39.7.
[0078] HRMS (ESI) for C 14 h 12 N 2 o 2 SNa [M+Na] + calc...
Embodiment 3
[0080]
[0081] Add 1-(3,4-dihydroquinolin-1-yl)-2,2-dimethylacetone 1c (43 mg, 0.2 mmol) to a 10 mL dry reaction tube, 1 mL of anhydrous dichloroethane , then added chlorosulfonyl isocyanate (70 mg, 0.5 mmol), and the mixture was stirred at 80 °C for 6 h. After the reaction mixture was concentrated under reduced pressure, it was separated by silica gel column chromatography (eluent was petroleum ether / ethyl acetate (v / v = 5 / 1-1 / 2)) to obtain 42 mg of the product 3-tert-butyl- 6,7-dihydro-5 H -1,2,4-Benzothiadiazine-1,1-dioxide 2c, 75% yield.
[0082] NMR data on product 2c as Figure 6~7 As shown, the details are as follows:
[0083] 1 H NMR (400 MHz, CDCl 3 ): δ 7.80 (d, J = 6.8 Hz, 1H), 7.40 (d, J = 6.8Hz, 1H), 7.35 (t, J = 7.6 Hz, 1H), 4.17–4.19 (m, 2H), 3.05 (t, J =6.4 Hz,2H), 2.16 (m, 2H), 1.50 (s, 9H).
[0084] 13 C NMR (100 MHz, CDCl 3 ): δ 167.3, 136.2, 133.4, 127.4, 126.1, 123.7, 122.3, 47.9, 40.6, 29.4, 27.1, 21.5.
[0085] HRMS (ESI) for C 14 h ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com