Large-steric-hindrance N-heterocyclic carbene palladium complex, preparation method and application thereof, and synthesis method of Sonidegib based on large-steric-hindrance N-heterocyclic carbene palladium complex
A complex and nitrogen heterocycle technology is applied in the preparation of amino compounds, the preparation of organic compounds, organic compounds/hydrides/coordination complex catalysts, etc. Fewer synthesis steps, avoiding palladium-carbon hydrogenation process, and efficient C-N coupling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
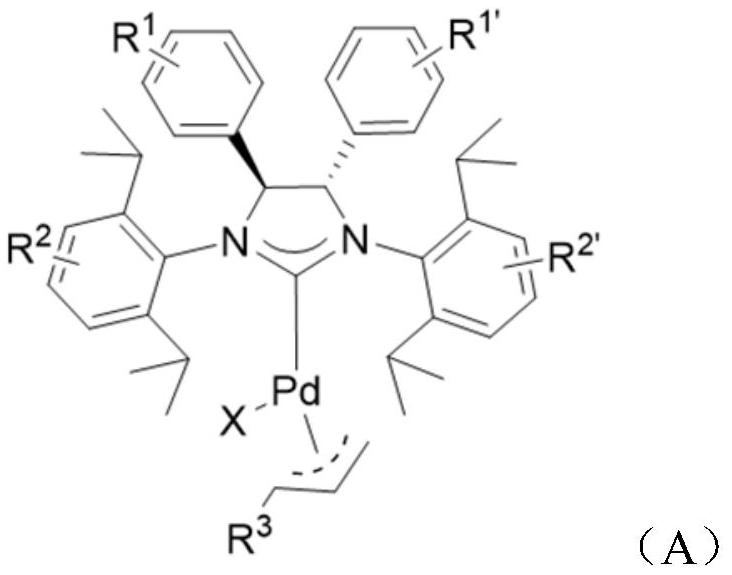
[0064] One embodiment, a large sterically hindered nitrogen heterocyclic carbene palladium complex (NHC-Pd complex), the structural formula is as follows:
[0065]
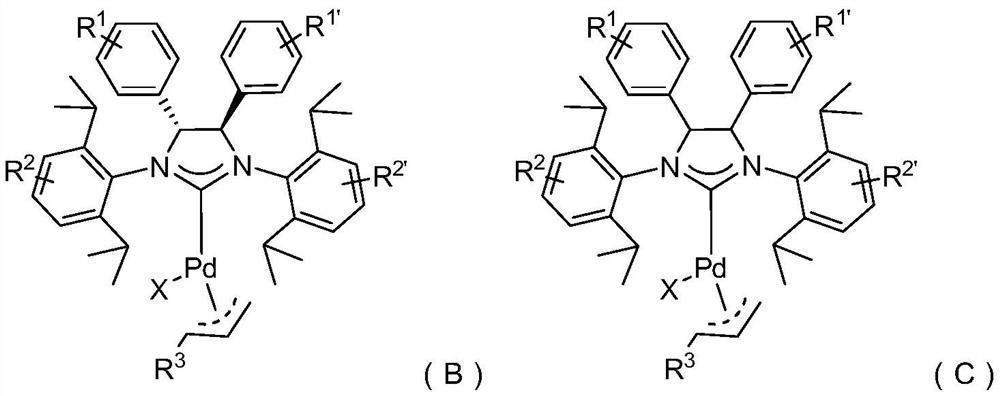
[0066] Another embodiment, a large sterically hindered nitrogen heterocyclic carbene palladium complex (NHC-Pd complex), the structural formula is as follows:
[0067]
[0068] Yet another embodiment, a large sterically hindered nitrogen heterocyclic carbene palladium complex (NHC-Pd complex), the structural formula is as follows:
[0069]
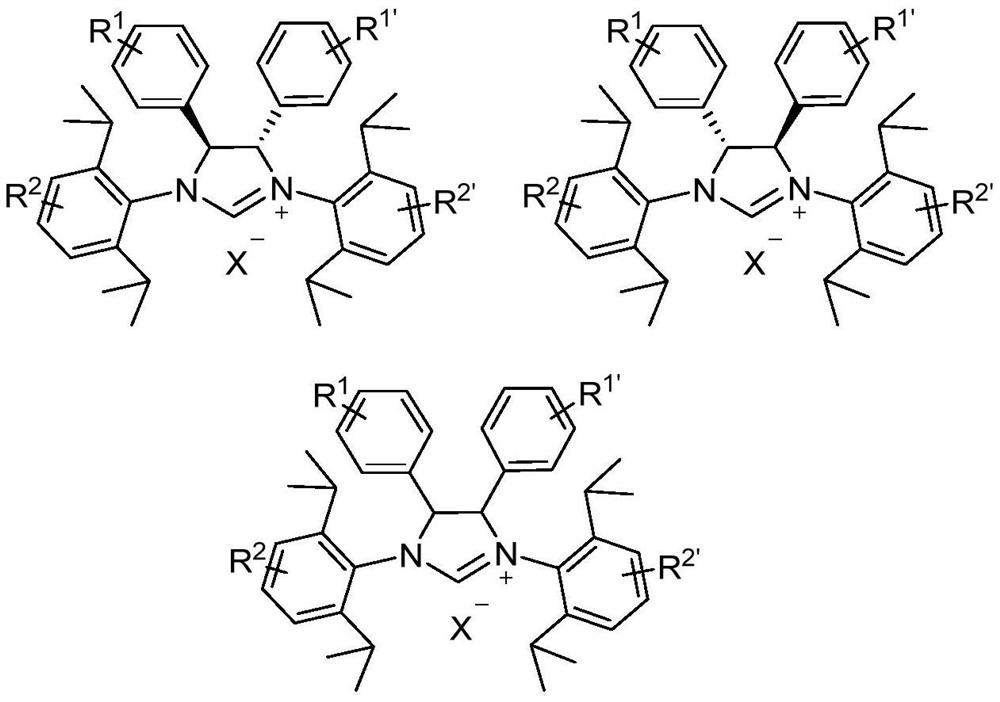
[0070] It should be noted that the large sterically hindered nitrogen heterocyclic carbene palladium complex structure of the present invention uses diphenylimidazole as the main ligand skeleton and functionalized allyl as auxiliary ligand. As an auxiliary ligand, the allyl group is not very tightly combined with the metal center, so it is easily activated into zero-valent palladium at room temperature, inserting into the C-Cl bond of the aromatic heterocyclic chloride,...
Embodiment 1
[0077] Embodiment 1: the synthesis of nitrogen heterocyclic carbene palladium complex Pd-NHC-1
[0078]
[0079] (1) Synthesis of diamine compound 2a:
[0080]
[0081] 5mmol of (1S,2S)-(-)-1,2-diphenylethylenediamine, 18mmol of tBuONa, 15mmol of 2,6-diisopropylbromobenzene, 1.5mmol of IPrMe·HCl, 0.5mmol Pd(dba) 2 Mix, add 20mL of anhydrous toluene, and react at 110°C for 24h under nitrogen atmosphere. After the reaction was completed, dilute with water, extract 3 times with ethyl acetate, anhydrous Na 2 SO 4 dry. The solvent was spin-dried under reduced pressure, and the crude product was subjected to silica gel column chromatography to obtain a white solid (eluent: petroleum ether / dichloromethane=10:1). Product yield: 1.70 g, yield: 65%. 1 H NMR (400MHz, CDCl 3 )δ7.06-6.99(m,16H),4.58(s,2H),4.24(s,2H),3.29(dt,J=13.1,6.4Hz,4H),1.23(d,J=6.6Hz,12H ),0.91(d,J=6.5Hz,12H). 13 C NMR (101MHz, CDCl 3 ) δ 143.1, 141.3, 140.2, 128.4, 127.8, 127.0, 123.7, 123.4, 69.1, 27...
Embodiment 2
[0092] Embodiment 2: the synthesis of nitrogen heterocyclic carbene palladium complex Pd-NHC-2
[0093]
[0094] (1) Synthesis of diamine compound 2b:
[0095]
[0096] 5mmol of (1S,2S)-(-)-1,2-diphenylethylenediamine, 18mmol of tBuONa, 15mmol of 2,4,6-triisopropylbromobenzene, 1.5mmol of IPrMe·HCl, 0.5mmol of Pd(dba) 2 Mix, add 20mL of anhydrous toluene, and react at 110°C for 24h under nitrogen atmosphere. After the reaction was completed, dilute with water, extract 3 times with ethyl acetate, anhydrous Na 2 SO 4 dry. The solvent was spin-dried under reduced pressure, and the crude product was subjected to silica gel column chromatography to obtain a white solid (eluent: petroleum ether / dichloromethane=10:1). Product yield: 2.15 g, yield: 70%. 1 H NMR (400MHz, CDCl 3 )δ7.10–6.94(m,10H),6.86(s,4H),4.57(d,J=4.3Hz,2H),4.18(s,2H),3.45–3.13(m,4H),2.95–2.63 (m,2H),1.33–1.12(m,24H),0.87(d,J=6.8Hz,12H). 13 C NMR (101MHz, CDCl 3 ) δ 143.7, 143.0, 140.5, 139.0, 128.5, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com