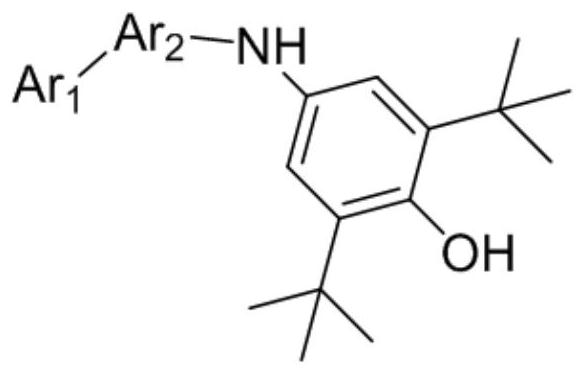
Phenol amine antioxidant as well as preparation method and application thereof
A technology of antioxidants and phenolic amines, applied in the field of antioxidants and their preparation, can solve the problems of few types of antioxidants and cannot meet diversified needs, and achieve low dyeing pollution, easy industrialization, and not easy to deteriorate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The synthetic route is as follows:
[0048]
[0049] The synthesis steps are as follows:
[0050] (1) Dissolve compound I (1mol) and compound II (1mol) in toluene, then add 2.5mol of Na 2 CO 3 The prepared 2mol / L aqueous solution and 0.06mol of Pd(PPh 3 ) 4 , after replacing the nitrogen three times, under the protection of nitrogen, react at 85°C for 6 hours;
[0051] (2) After the reaction is completed, cool to room temperature, add water to wash, take the upper organic phase, dry it with anhydrous magnesium sulfate, and remove the solvent by rotary evaporation, then pass through 200-300 mesh silica gel column chromatography, and the eluent composition is volume ratio 2 Chloromethane pure: petroleum ether = 3: 1, to obtain compound III;
[0052] (3) Compound III (1mol), Pd / C catalyst (50g) and N 2 h 4 -H 2 O (hydrazine hydrate, 200ml) was dissolved in EtOH, and reacted at 60°C for 2 hours. After the reaction, the Pd / C catalyst was removed by suction filtrat...
Embodiment 2
[0056] Synthetic route is with embodiment 1, and synthetic steps are as follows:
[0057] (1) Dissolve compound I (1mol) and compound II (1mol) in dioxane, then add 2.5mol of K 2 CO 3 Prepared 2mol / L aqueous solution and 0.06mol PdCl 2 , after replacing the nitrogen three times, under the protection of nitrogen, react at 85°C for 6 hours;
[0058] (2) After the reaction is completed, cool to room temperature, add water 3-5 times the volume of the reaction solution, and precipitate a large amount of crude product. After filtering, dissolve in dichloromethane, dry with anhydrous magnesium sulfate, and pass through 200-300 mesh silica gel Column chromatography, the eluent composition is pure by volume ratio of dichloromethane:petroleum ether=3:1, to obtain compound III;
[0059] (3) Compound III (1mol), Pd / C catalyst (50g) and N 2 h 4 -H 2 O (hydrazine hydrate, 200ml) was dissolved in EtOH, and reacted at 60°C for 2 hours. After the reaction, the Pd / C catalyst was removed b...
Embodiment 3
[0063] The synthetic route is as follows:
[0064]
[0065] The synthesis steps are as follows:
[0066] (1) Dissolve compound I (1mol) and compound II (1mol) in toluene or dioxane, then add 2mol of Cs 2 CO 3 Prepared 2mol / L aqueous solution and 0.03mol Pd(dppf)Cl 2 , after replacing the nitrogen three times, under the protection of nitrogen, react at 70°C for 8 hours;
[0067] (2) After the reaction is completed, cool to room temperature, add water to wash, take the upper organic phase, dry with anhydrous sodium sulfate, and remove the solvent by rotary evaporation, then pass through 200-300 mesh silica gel column chromatography, and the eluent composition is volume ratio 2 Chloromethane pure: petroleum ether = 3: 1, to obtain compound III;
[0068] (3) Compound III (1mol), Pd / C catalyst (30g) and N 2 h 4 -H 2 O (hydrazine hydrate, 150ml) was dissolved in EtOH, and reacted at 55°C for 3h. After the reaction, the Pd / C catalyst was removed by suction filtration, dried...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com