Preparation method of o-aryl primary amide
A technology of primary amide and ortho-aryl, which is applied in the field of preparation of primary aryl amide, can solve the problems of ineffectiveness of primary aryl amide, low functional group compatibility, and difficulty in separating carboxylic acid, and achieves a wide range of substrates, The effect of low dosage and extensive functional group transformation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The preparation method of o-aryl primary amides provided by the present invention uses α-silyl nitrile and aryl sulfoxide as substrates, and in the presence of acid and solvent, [3,3]-Sigmatropic rearrangement reaction occurs at 50°C to obtain The product ortho-aryl primary amide solves the problem that carboxylic acid by-products or nitrogen arylation products are easily produced in the prior art.
[0034] The preparation method provided by the invention has high chemoselectivity and does not produce carboxylic acid and nitrogen arylation by-products which are difficult to separate. The prepared ortho-aryl primary amide compounds have great application value, and can be further extensively transformed to prepare ketones, esters, carboxylic acids, amines, nitrogen heterocycles, etc., which are of great significance in drug synthesis.
[0035] The raw materials involved in each embodiment of the present invention are either existing commercially available products, or ca...
Embodiment 1
[0037] Preparation of o-aryl primary amides
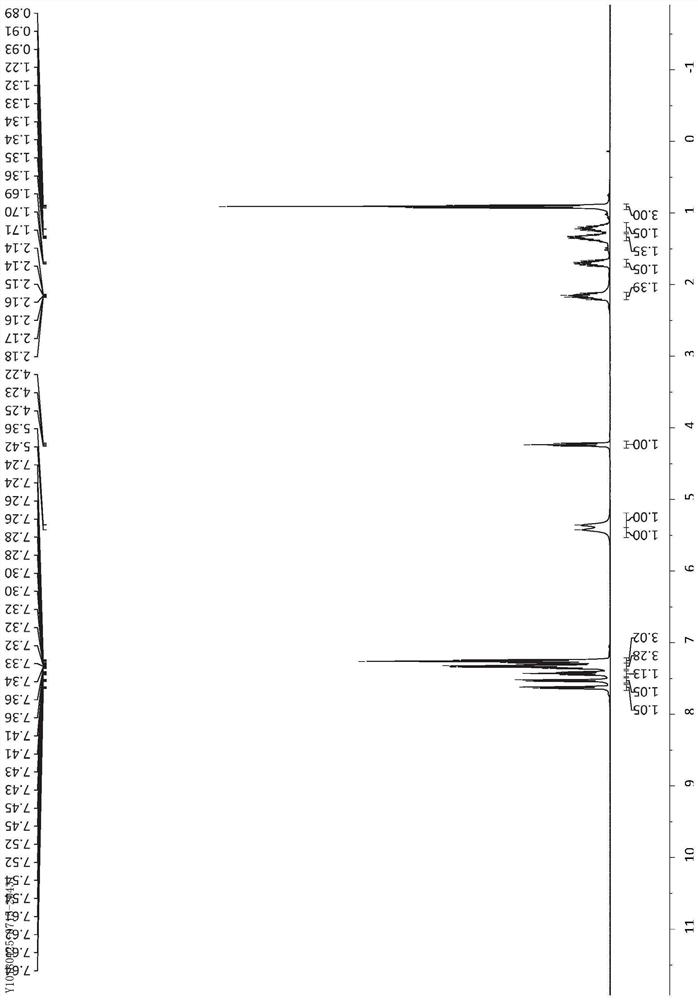
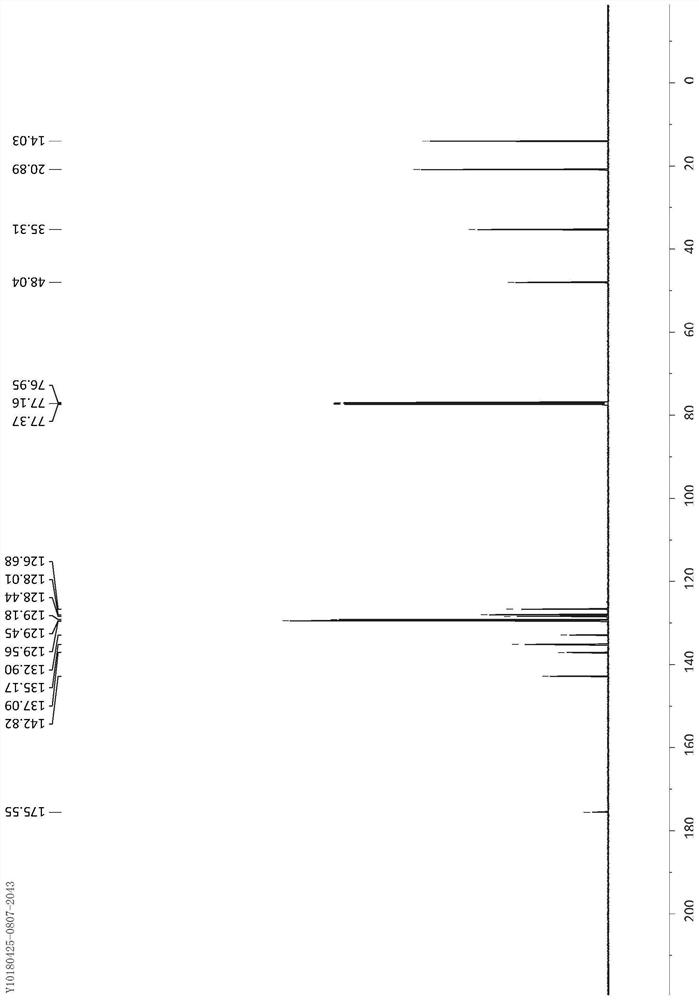
[0038] Dissolve α-silylnitrile 1 (0.75mmol) and arylsulfoxide 2 (0.5mmol) in DCE (3.3mL), slowly add TfOH (67μL, 0.75mmol) dropwise at 0°C, and continue stirring for 10 minutes after dropping , followed by heating to 50°C and stirring for 12 hours. After returning to room temperature, a saturated sodium bicarbonate solution (5 mL) was added to the reaction solution to terminate the reaction, the mixture was extracted with DCM (5 mL×3), the extracts were combined, dried over sodium sulfate, concentrated under reduced pressure, and the residue was washed with silica gel powder The column yielded the aryl primary amide product 3.
[0039] The different reaction substrates α-silyl nitrile 1 and aryl sulfoxide 2 and the obtained corresponding product aryl primary amide product 3 are as follows:
[0040]
[0041]
[0042] The above-mentioned yield is the separation yield, and the time for the completion of the reaction is 12 hour...
Embodiment 2
[0179]
[0180] Dissolve α-silylnitrile 1a (0.75mmol) and arylsulfoxide 2a (0.5mmol) in DCE (3.3mL), slowly add Tf dropwise at 0°C 2 NH (0.75mmol), continue to stir for 10 minutes after dropping, then raise the temperature to 50°C and stir for 12 hours. After returning to room temperature, a saturated sodium bicarbonate solution (5 mL) was added to the reaction solution to terminate the reaction, the mixture was extracted with DCM (5 mL×3), the extracts were combined, dried over sodium sulfate, concentrated under reduced pressure, and the residue was washed with silica gel powder The aryl primary amide product 3a was obtained by the column, and the separation yield was 65%.
[0181]Dissolve α-silylnitrile 1a (0.75mmol) and aryl sulfoxide 2a (0.5mmol) in DCE (3.3mL), slowly add TfOH (0.75mmol) dropwise at 0°C, continue stirring for 10 minutes after the drop is complete, and then The temperature was raised to 40°C and stirred for 12 hours. After returning to room temperatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com