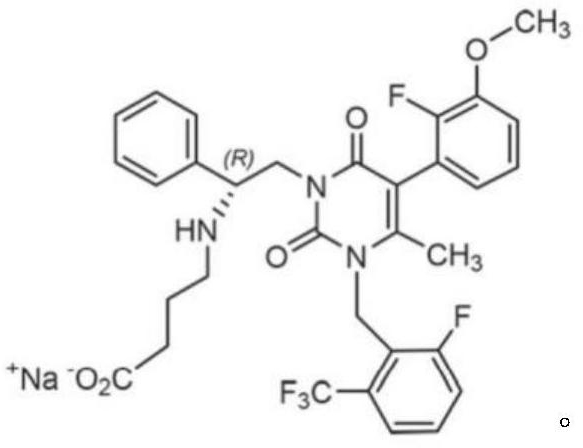
Elagolix freeze-dried tablets and preparation method thereof
A technology of freeze-dried tablets and solvents, which is applied in the field of medicine, can solve the problems of high protection requirements for operators, high production environment requirements, and inevitable environmental pollution, and achieve rapid clinical effect, low water content, and low environmental pollution. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The preparation technology of elagoli freeze-dried tablet of the present invention:
[0034] 1) The raw materials are crushed mechanically, such as universal grinder, hammer mill, ball star grinder, etc., until the particle size reaches D90<10μm, D50≈5μm;
[0035] 2) Weigh the material according to the prescription amount;
[0036] 3) raw material solution: add the raw material and anhydrous sodium carbonate to 50% purified water (50% of the total prescription of purified water), stir and dissolve at room temperature;
[0037] 4) Excipient solution: dissolve povidone in 40% purified water (40% of the total prescription volume of purified water), then add xylitol, sucralose and essence, and stir and mix evenly at 30-35°C;
[0038] 5) Homogenization: Mix the raw material solution and the povidone auxiliary material solution, and use a homogenizer to homogenize at 2000 rpm for 20 to 30 minutes;
[0039] 6) Constant volume: add purified water to constant volume, and conti...
Embodiment 1
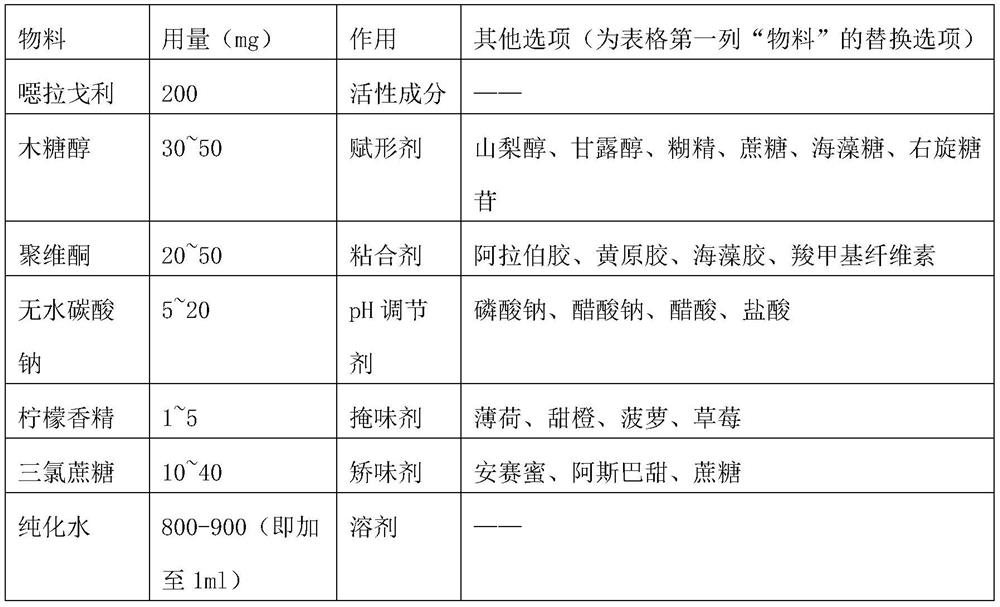
[0046] Materials are shown in the table below.
[0047] materials Dosage (mg) Ella Goli 200 Xylitol 30 povidone 20 Anhydrous Sodium Carbonate 10 lemon zest 3 Sucralose 20 purified water Add to 1ml
[0048] 1) The raw materials are crushed mechanically, such as universal grinder, hammer mill, ball star grinder, etc., until the particle size reaches D90<10μm, D50≈5μm;
[0049] 2) Weigh the material according to the prescription amount;
[0050] 3) raw material solution: add the raw material and anhydrous sodium carbonate to 50% purified water, stir and dissolve at room temperature;
[0051] 4) Excipient material solution: dissolve povidone in 40% purified water, then add xylitol, sucralose and essence, stir and mix evenly at 30-35°C;
[0052] 5) Homogenization: mix the raw material solution and povidone solution, and use a homogenizer to homogenize at 2000 rpm for 20 to 30 minutes;
[0053] 6) Constant volume: add puri...
Embodiment 2
[0060] Materials are shown in the table below.
[0061] materials Dosage (mg) Ella Goli 200 Xylitol 50 povidone 30 Anhydrous Sodium Carbonate 5 lemon zest 1 Sucralose 10 purified water Add to 1ml
[0062] 1) The raw materials are crushed mechanically, such as universal grinder, hammer mill, ball star grinder, etc., until the particle size reaches D90<10μm, D50≈5μm;
[0063] 2) Weigh the material according to the prescription amount;
[0064] 3) raw material solution: add the raw material and anhydrous sodium carbonate to 50% purified water, stir and dissolve at room temperature;
[0065] 4) Excipient material solution: dissolve povidone in 40% purified water, then add xylitol, sucralose and essence, stir and mix evenly at 30-35°C;
[0066] 5) Homogenization: mix the raw material solution and povidone solution, and use a homogenizer to homogenize at 2000 rpm for 20 to 30 minutes;
[0067] 6) Constant volume: add purif...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com