Method for preparing hesperetin dihydrochalcone glucoside by immobilized enzyme method
A technology of hesperetin dihydrochalcone and hesperidin dihydrochalcone, which is applied in the direction of immobilized enzymes, biochemical equipment and methods, glycosylases, etc., can solve the difficulty of separation and purification of the target product, difficult Realize the problems of industrialized production, HSD hydrolysis by-products, etc., to achieve the effect of low cost, good stability, and reduced energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
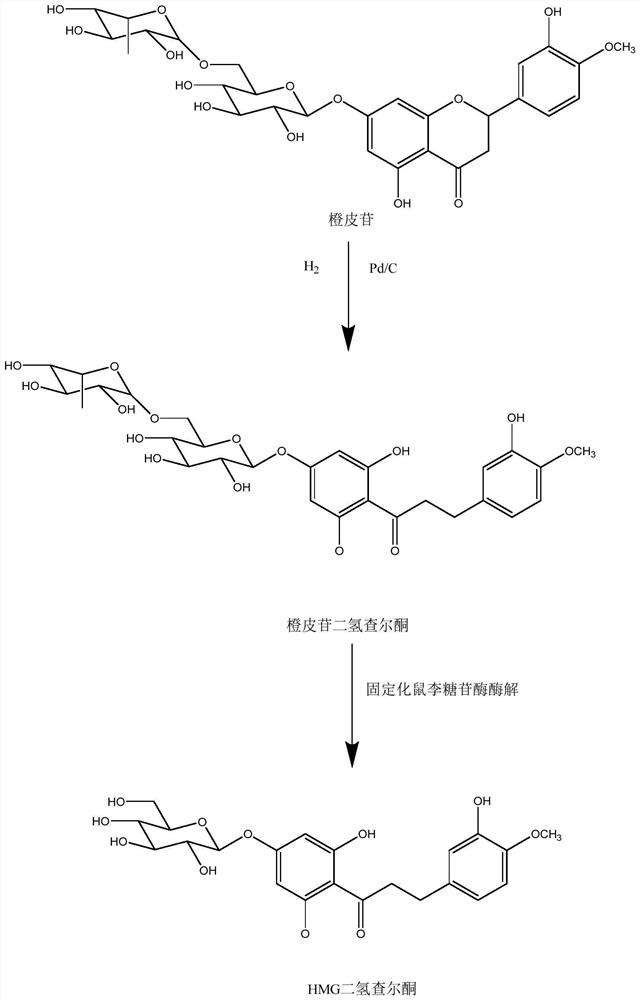
[0049] Such as figure 1 As shown, the immobilized enzymatic conversion method for realizing hesperidin to hesperetin dihydrochalcone glucoside is mainly divided into the following three steps: (1) catalytic hydrogenation of hesperidin; (2) immobilized enzyme Preparation; (3) Enzymatic hydrolysis of the hydrogenation product of hesperidin to synthesize hesperetin dihydrochalcone glucoside. The specific operation is:
[0050] (1) Accurately weigh 10 g of hesperidin, 30 g of sodium hydroxide, and 5 g of Raney nickel. Dissolve in 100mL deionized water, add the solution to the reaction kettle, and pass N 2 , and then evacuate and feed N 2 , and then evacuate and then feed H 2 , repeated into H 2 React for about 1 to 6 hours until the H in the reactor 2When the pressure does not change any more, the reaction is terminated, the solution is transferred to a beaker, and 37% concentrated hydrochloric acid is added to adjust the pH to about 3. Take 1mL of the solution and filter i...
Embodiment 2
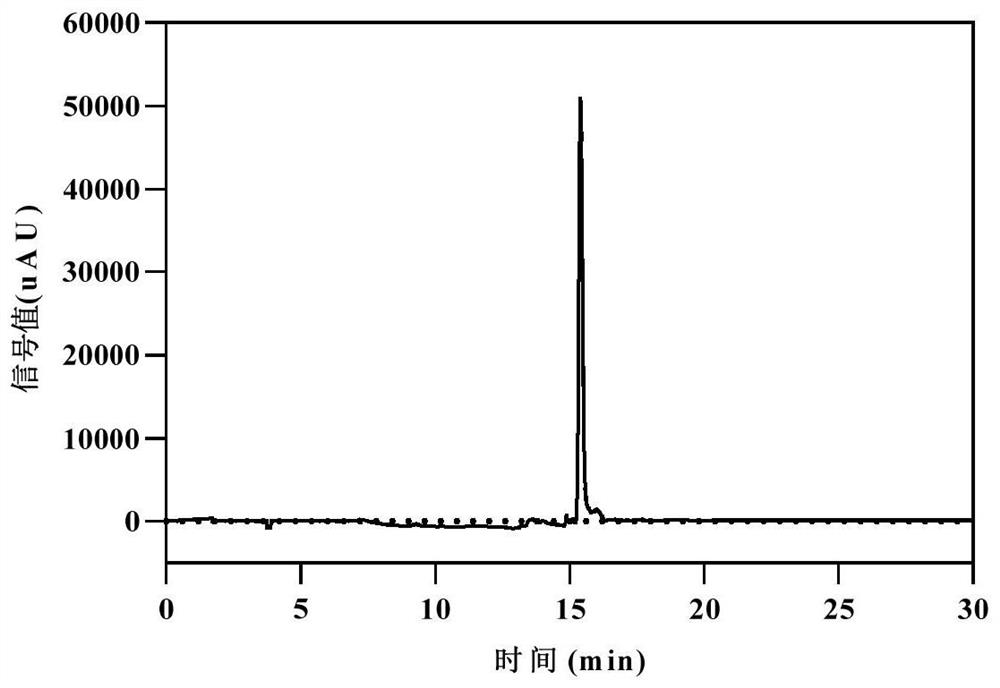
[0054] (1) Accurately weigh 50 g of hesperidin, 15 g of sodium hydroxide, and 25 g of Raney nickel. Dissolve in 500mL deionized water, add the solution to the reaction kettle, and pass N 2 , and then evacuate and feed N 2 , and then evacuate and then feed H 2 , repeated into H 2 React for about 1 to 6 hours until the H in the reactor 2 The pressure does not change anymore, the reaction is terminated, the solution is transferred to a beaker, and concentrated hydrochloric acid is added to adjust the pH to about 3. Take 1mL of the solution and filter it with a 0.22μm microporous membrane. Take 20 μL of the filtered sample solution and directly inject it into the HPLC system to analyze the product hesperidin dihydrochalcone.
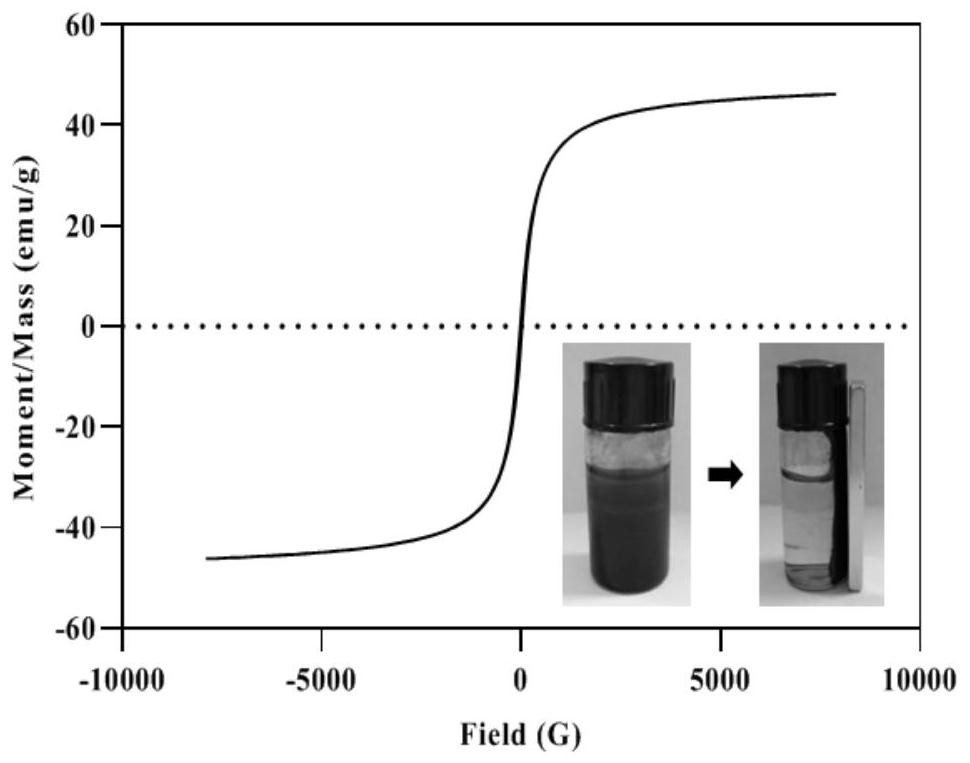
[0055] (2) Take 100g MIL-101(Cr) and disperse it in water evenly, and add 200g FeCl 3 ·6H 2 O and 100g FeSO 4 ·7H 2 O, after stirring and dissolving, raise the temperature to 85°C and continue to stir for 30 minutes, add ammonia dropwise to adjust t...
Embodiment 3
[0058] (1) Accurately weigh 30 g of hesperidin, 20 g of sodium hydroxide, and 10 g of Raney nickel. Dissolve in 300mL deionized water, add the solution to the reaction kettle, and pass N 2 , and then evacuate and feed N 2 , and then evacuate and then feed H 2 , repeated into H 2 React for about 1 to 6 hours until the H in the reactor 2 The pressure does not change anymore, the reaction is terminated, the solution is transferred to a beaker, and concentrated hydrochloric acid is added to adjust the pH to about 3. Take 1mL of the solution and filter it with a 0.22μm microporous membrane. Take 20 μL of the filtered sample solution and directly inject it into the HPLC system to analyze the product hesperidin dihydrochalcone.
[0059] (2) Disperse 80g MIL-101(Cr) in water evenly, and add 300g FeCl 3 ·6H 2 O and 150gFeSO 4 ·7H 2 O, after stirring and dissolving, raise the temperature to 65°C and continue stirring for 30 minutes, add ammonia water dropwise to adjust the pH t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com