Preparation method and application of bimetallic Prussian blue analogue catalyst
A Prussian blue and catalyst technology, which is applied in the field of preparation of bimetallic Prussian blue analog catalysts, can solve the problems of poor stability, limited number of catalyst active sites, and low utilization rate of oxidants, and achieve good cycle stability and broaden the Response pH range, low toxicity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
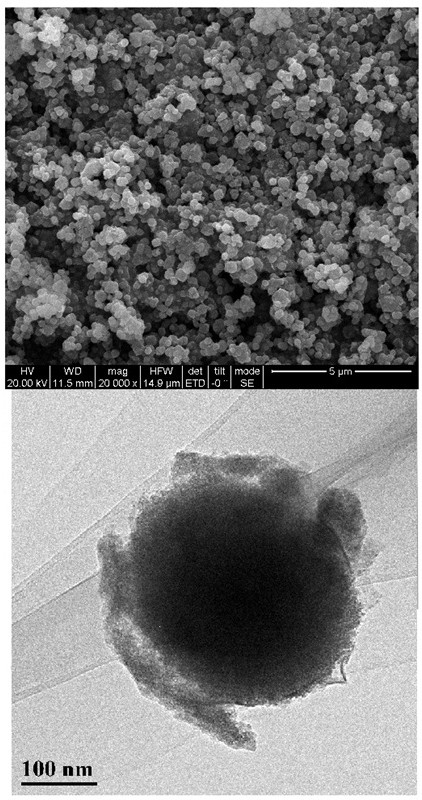
Image
Examples
Embodiment 1
[0050] 1) Weigh 1.8 g of polyvinylpyrrolidone and fully dissolve it in 60 mL of water, add 73.944 mg (5 mM) of anhydrous cerium chloride and mix with the above solution to prepare dispersion A, weigh 98.775 mg (5 mM) Potassium ferricyanide was added into 60 mL of water, and stirred ultrasonically until the potassium ferricyanide was uniformly dissolved to obtain solution B (the molar ratio of the bimetallic precursor was 1:1);
[0051] 2) Dispersion A was slowly dripped into solution B drop by drop to undergo cation exchange reaction to obtain suspension C, and its pH was adjusted to 4 with 1 M dilute hydrochloric acid; the suspension C was transferred to a three-necked flask, ℃, 2000 rpm stirring and heating conditions for hydrothermal reaction, the reaction time was controlled at 20 h, and precipitate D was formed.
[0052] 3) After centrifugation, the obtained precipitate was washed three times with ethanol and water by suction filtration, and finally dried at 60°C for 12 h...
Embodiment 2
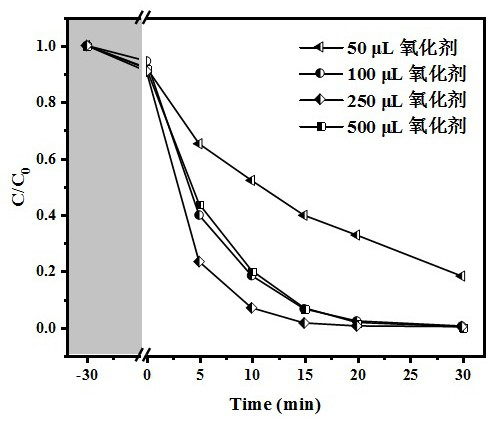
[0057] The difference between this embodiment and Example 1 is that the ratio of the amount of anhydrous cerium chloride and potassium ferricyanide in step 1) is 2:1, 3:2, 1:1, 2: 3. 1:2, the others are the same as in Example 1.
[0058] See the experimental results figure 2 ; Under the condition of the optimal bimetallic precursor ratio (1:1), the catalyst can achieve the complete degradation of sulfamethoxazole within 30 min, and has obvious kinetic advantages.
Embodiment 3
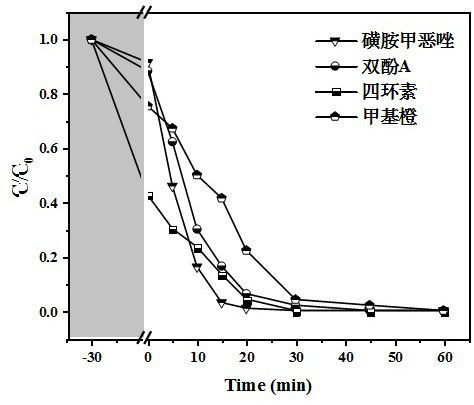
[0060] The difference between this embodiment and Example 1 is that in step 2), 1 M dilute hydrochloric acid is used to adjust the material synthesis pH to 2, 3, 4, and 5, and the others are the same as Example 1.
[0061] See the experimental results image 3 The synthesis pH affects the crystal nucleation and growth rate of the catalyst. Under the optimal pH of 4, the complete degradation of sulfamethoxazole can be achieved within 30 min, and has obvious kinetic advantages.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com