Application of mannose glucuronic acid oligosaccharides and polysaccharides and derivatives in preparation of drugs for treating and/or preventing aging
A technology of uronic acid oligosaccharide and uronic acid polysaccharide, applied in the field of pharmacy, can solve problems such as large side effects and general effects, and achieve the effects of small toxic side effects, safe sources and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: Preparation of mannoglucuronide oligosaccharides
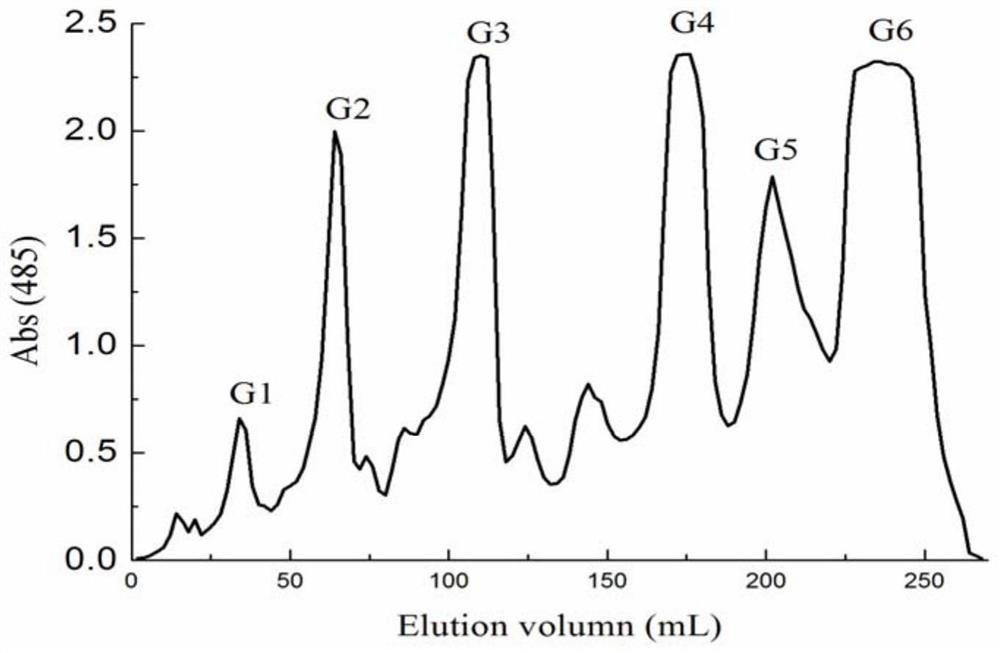
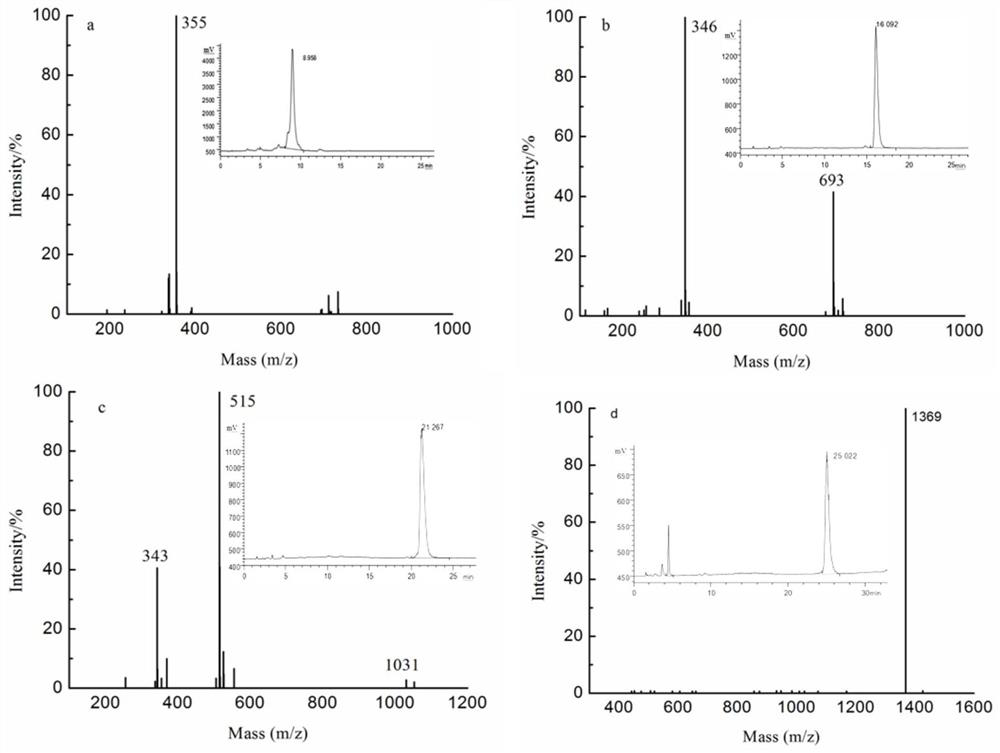
[0049] Dried kelp and other brown algae were extracted with 30 times the mass of distilled water at 100°C for 3 hours to remove the alginate, the extract was filtered, dialyzed, concentrated, added ethanol to a final concentration of 75% and precipitated, and the precipitate was collected after standing for 12 hours , sink to obtain fucoidan sulfate ester by vacuum drying. Dissolve the fucoidan sulfate sample in a sulfuric acid solution with a mass concentration of 4% (the ratio of solid to liquid is 60mg / mL), heat and reflux for 5 hours, neutralize with barium hydroxide to PH=6-7, centrifuge, and concentrate the supernatant To one-fifth of the original volume, the concentrated solution was subjected to active carbon column chromatography, firstly equilibrated with distilled water, and then eluted with a gradient of 50%-90% ethanol, and the 50%-90% ethanol eluate was concentrated to five parts of the origi...
Embodiment 2
[0055] Embodiment 2: Preparation of mannoglucuronide polysaccharide
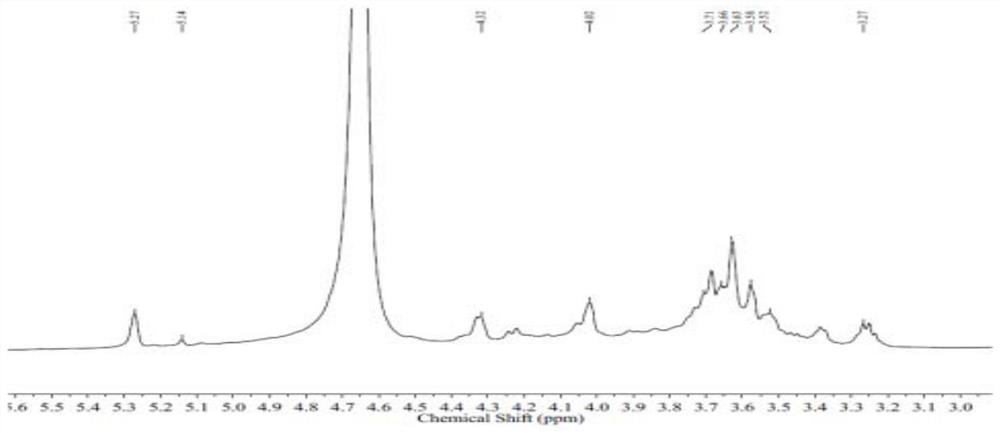
[0056] Dissolve the fucoidan sulfate obtained in Example 1 in a sulfuric acid solution with a mass concentration of 4% (the ratio of solid to liquid is 60 mg / mL) and heat to reflux for 4 hours, neutralize with barium hydroxide to PH=6-7, and centrifuge , the supernatant was concentrated to a small volume, and the concentrated solution was loaded on DEAE Sepharose Fast Flow (DEAE Sepharose gel, washed with water, 0.2M sodium chloride and 2M sodium chloride were eluted, and the 2M eluent was prepared by dialysis of a 500Da dialysis bag Obtain mannose glucuronide polysaccharide GMn (7.0kDa), the sample is carried out 1 H-NMR, 13 C-NMR, two-dimensional NMR and GPC-HPLC, etc. like image 3 and Figure 4 as shown, 1 H-NMR, 13 C-NMR is hydrogen nuclear magnetic resonance spectrum and carbon 13 nuclear magnetic resonance spectrum respectively. like Figure 5 As shown, the two-dimensional HMBC (1H detected he...
Embodiment 3
[0059] Example 3: Mannoglucuronide poly(oligo)saccharides can alleviate the decline in the viability of β cells caused by hydrogen peroxide
[0060] Treat MIN6 cells with mannose glucuronide poly(oligo)saccharides of the present invention at a concentration of 100 μg / ml, and the cell viability is not affected, indicating that the concentration has no cytotoxic effect ( Figure 9 A); then mannoglucuronide poly(oligo)saccharides are intervened with H 2 o 2 After pretreatment of islet β cells for 24 hours, mannoglucuronide poly(oligo)saccharides had a significant enhancement effect on cell viability ( Figure 9 B), if Figure 9 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com