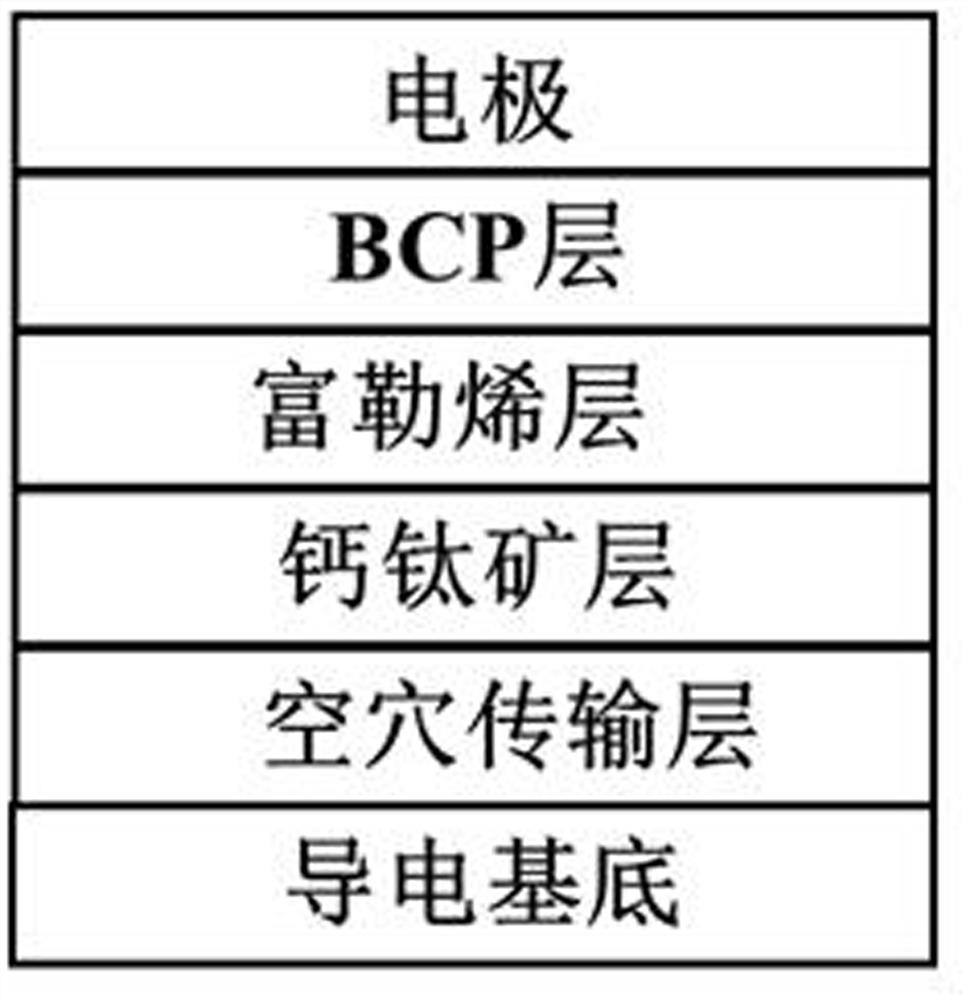
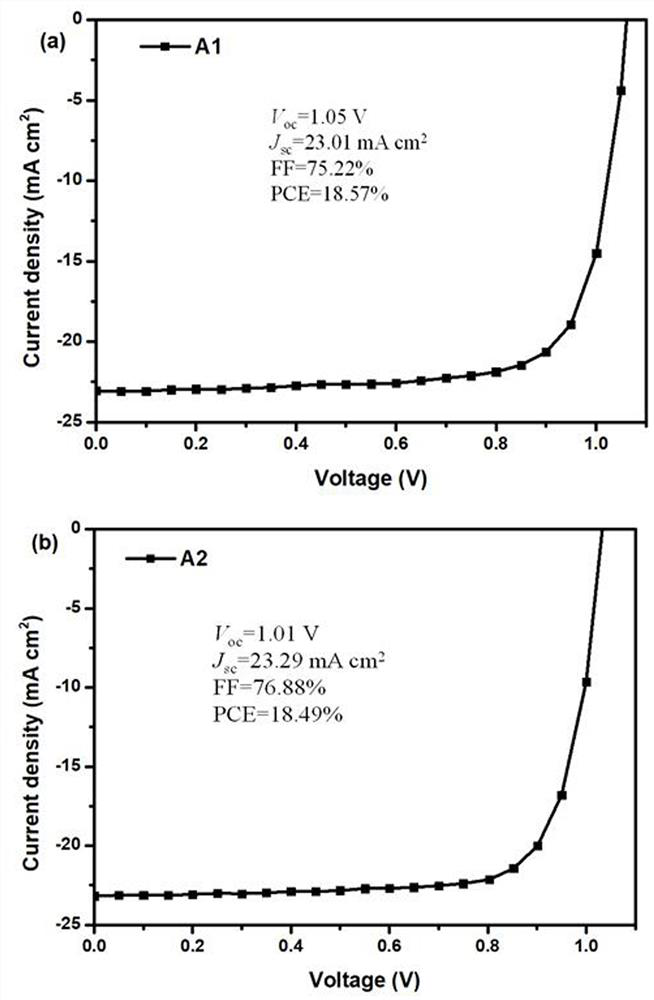
Triphenylamine fullerene derivative and preparation method and application thereof
A technology similar to fullerene and triphenylamine, which is applied in semiconductor/solid-state device manufacturing, photovoltaic power generation, electrical components, etc., can solve the problems of cumbersome preparation methods of electron transport materials, complex titanium dioxide production process, and difficult product purification and treatment, and achieve reaction The effect of cheap raw materials, strong extraction and transmission capabilities, and easy access to reaction raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation of trihenylamine fullerene derivatives-C 60 -fused n- (2-cyanoethyl) pyrrolidine-4- (di-p-tolylamino) Phenyl, C 60 And N- (2-cyanoethyl) pyrrolidine-4- (two-pactorino-amino) phenylfalleylene derivative (A1) method and product characterization:
[0044] Proceed as follows:
[0045] 1) Weigh 4-Methyl-N-Phenyl-N- (p-Tolyl) Aniline (5mmol, 1.0eq), using double-drain-tube vacuum filled with nitrogen, reaches the reaction environment of the reaction, add 10.0 to the system ML super dry DMF, place the reaction device in a low temperature cooling circulation device to cool to 0 ° C, then POCL 3 (7.5mmol, 1.5 eq) Added, after the addition is completed, transfer the reaction device into the oil bath, slowly warmed to 75 ° C, reaction 12h, stop the reaction, to be cooled to room temperature, to add excess water to the system to terminate the reaction , Extract three times with EtOAc, no water MGSO 4 Dry, filtration was concentrated, purified with silica gel column chromato...
Embodiment 2
[0051] Preparation of trihenylamine fullerene derivatives-C 60 -fused n- (2-cyanoethyl) Pyrrolidine-4- (Diphenylamino) Phenyl, C 60 The method and product characterization of N- (2-cyanoethyl) pyrrolidine-4- (diphenone) phenylllerene derivative (A2):
[0052] Proceed as follows:
[0053] 1) We will weigh the triphenylamine (5mmol, 1.0 eq), repeated three times with double-drain vacuum, reaches the reaction environment of the reaction, add 10.0 ml of super-oxygen-free DMF to the system, and place the reaction device at low temperature cooling. Cool to 0 ° C in the circulation device, then POCL 3 (7.5mmol, 1.5 eq) Added, after the addition is completed, transfer the reaction device into the oil bath, slowly warmed to 75 ° C, reaction 12h, stop the reaction, to be cooled to room temperature, to add excess water to the system to terminate the reaction , Extract three times with EtOAc, no water MGSO 4 Dry, filtration was concentrated, purified with silica gel column chromatography to o...
Embodiment 3
[0059] Preparation of trihenylamine fullerene derivatives-C 60 -fused n- (2-cyanoethyl) Pyrrolidine-4- (Bis (4-Methoxyphenyl) Amino) Phenyl, C 60 And N- (2-cyanoethyl) pyrrolidine-4- (two-pactoxyenenenenenen) phenylfolhaleylene derivative (A3) method and product characterization:
[0060] Proceed as follows:
[0061] 1) Weigh aniline (1 mmol, 1.0 eq), 8-hydroxyquinoline (0.05 mmol, 0.05 eq), K 3 PO 4 (4 mmol, 4.0 eq), Cucl (0.05mmol, 0.05 eq), 1-IODO-4-Methoxybenzene (2.5mmol, 2.5 eq) in two flasks, construction of the ball gallbladder reflow closing reaction device, using double-distance tube The vacuum nitrogen gas was repeated three times, and 10 ml of super dry DMF reflux was added to the system, and the reaction was filtered, washed with dichloromethane, concentrated to dryness, purified with silica gel column chromatography to obtain a target product 4-Methoxy -N- (4-methoxyphenyl) -n-Phenylaniline;
[0062] 2) Weigh 4-methoxy-N- (4-methodoxyphenyl) -N-Phenylaniline (1 mmol,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com